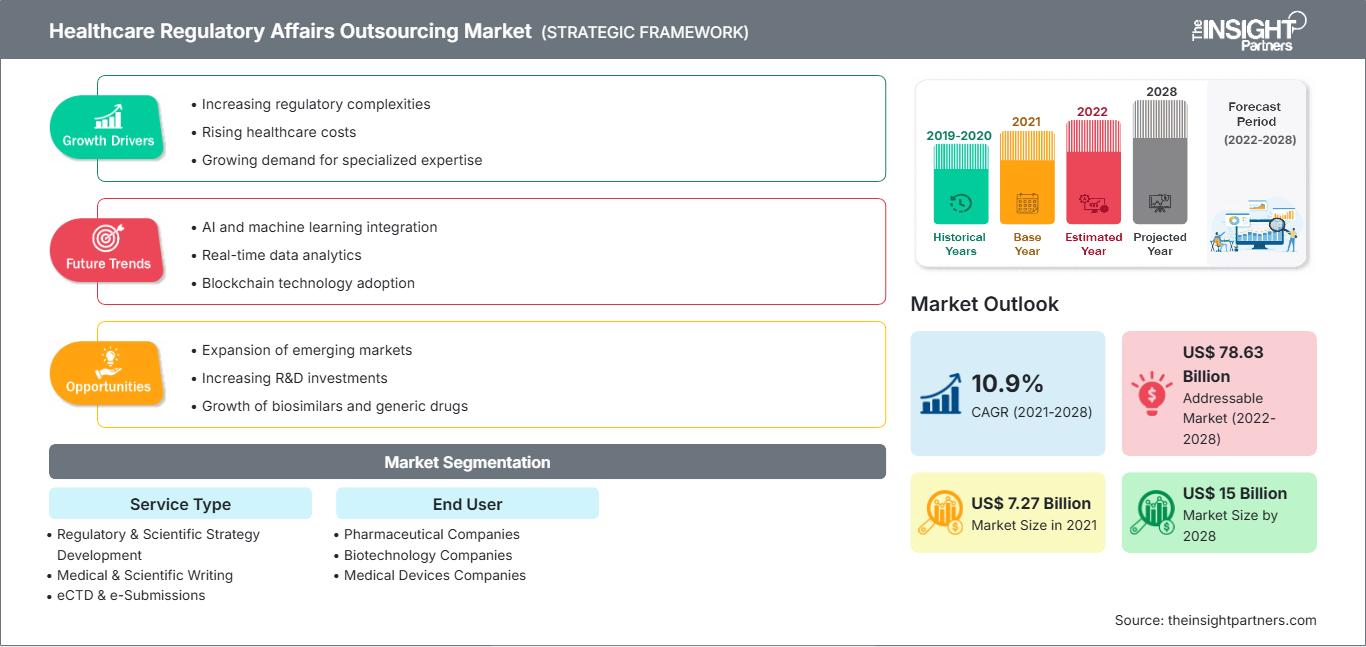
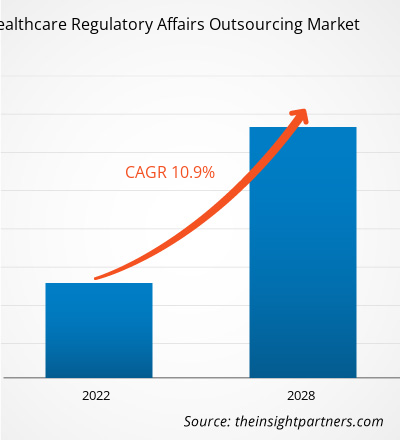
[Informe de investigación] Se proyecta que el mercado de subcontratación de aviones regulatorios atención de alcance médico los US$ 14.996,35 millones para 2028 desde los US$ 7.274,73 millones en 2021; se espera que crezca a una CAGR del 10,9 % entre 2021 y 2028.
La creciente presión regulatoria sobre las empresas del sector salud y la creciente demanda de aprobación rápida de nuevos productos. Sin embargo, la escasez de profesionales cualificados está frenando el crecimiento del mercado de externalización de asuntos regulatorios en el sector salud. La externalización de asuntos regulatorios consiste en servicios ofrecidos a las industrias farmacéutica, biotecnológica y de fabricación de dispositivos médicos. Estos servicios ayudan a obtener aprobaciones regulatorias rápidas. Las industrias que la subcontratan ayudan a obtener la aprobación de nuevos productos, preparar protocolos para la realización de ensayos clínicos y publicar informes, entre otros. El aumento de la demanda de diversos servicios, como la consultoría regulatoria, la redacción y publicación de documentación regulatoria, las solicitudes de ensayos clínicos, la consultoría regulatoria y la representación legal, la solicitud de patentes, el registro de productos y las solicitudes de ensayos clínicos, ha provocado un aumento en la adopción de la externalización de asuntos regulatorios en el sector salud.
Personalice este informe según sus necesidades
Obtendrá personalización en cualquier informe, sin cargo, incluidas partes de este informe o análisis a nivel de país, paquete de datos de Excel, así como también grandes ofertas y descuentos para empresas emergentes y universidades.
Mercado de externalización de asuntos regulatorios de salud: Perspectivas estratégicas
-
Obtenga las principales tendencias clave del mercado de este informe.Esta muestra GRATUITA incluye análisis de datos, desde tendencias del mercado hasta estimaciones y pronósticos.
Perspectivas del mercado
Aumento de la presión regulatoria sobre las empresas de atención médica Crecimiento del mercado de subcontratación de asuntos regulatorios de atención médica
Las continuas mejoras y avances en los enfoques tradicionales de desarrollo de fármacos están creando importantes desafíos en el sector sanitario. Existe una enorme presión sobre las compañías farmacéuticas y la comunidad médica para reducir el coste de los medicamentos con receta, mientras que sus costes operativos se disparan. La complejidad de los requisitos regulatorios, la disminución de los ingresos debido a la caducidad de patentes de medicamentos de gran éxito y la presión de los gobiernos y las aseguradoras de salud para reducir los costes sanitarios han presentado desafíos adicionales para las industrias sanitarias. Ante estas dificultades, las compañías farmacéuticas se han dado cuenta de la necesidad de aprovechar sus recursos junto con la experiencia de fuentes externas especializadas. Muchas empresas de consultoría regulatoria de alto nivel ofrecen su experiencia a lo largo de todo el ciclo de vida del producto. La externalización de los asuntos regulatorios puede permitir a los patrocinadores adquirir experiencia, optimizar costes y mejorar la productividad. Las empresas de externalización regulatoria están en mejor posición para evaluar los requisitos regulatorios, lo que les permite seleccionar las mejores soluciones. Poseen un profundo conocimiento de la implementación, operación y mantenimiento de un sistema de publicación regulatoria. La mayoría de las grandes compañías farmacéuticas y biotecnológicas buscan empresas de consultoría que también puedan ofrecer servicios de apoyo regulatorio y de farmacovigilancia.
La creciente complejidad de los trámites regulatorios subraya la demanda de experiencia especializada de CRO. Contar con asesoramiento y estrategias regulatorias específicas para cada producto, junto con medidas de cumplimiento regulatorio en el sector salud, planificadas en las primeras etapas del desarrollo del producto, es extremadamente importante para la aprobación regulatoria de los productos. No abordar el cumplimiento en la etapa temprana del desarrollo a menudo conduce a retrasos en el proceso de aprobación debido a documentación presentada incorrectamente, descuidos en la fabricación, estudios regulatorios omitidos y otros incumplimientos de los requisitos regulatorios. Las empresas del sector salud ahora se están enfocando en sus competencias principales y externalizando las funciones no esenciales para mejorar la productividad y la eficiencia operativa. Generalmente externalizan las funciones regulatorias a CRO que operan en mercados emergentes, como Asia Pacífico y Oriente Medio y África, lo que también les permite reducir sus costos operativos y fortalecer su enfoque en funciones principales como las actividades de I+D y la venta y distribución de productos existentes.
Información basada en el tipo de servicio
Según el tipo de servicio, el mercado de externalización de asuntos regulatorios en el sector salud se segmenta en Desarrollo de Estrategias Regulatorias y Científicas, Redacción Médica y Científica, eCTD y Envíos Electrónicos, Servicios de Gestión de Datos, Servicios de Gestión del Ciclo de Vida, Farmacovigilancia, Servicios de Fabricación y Control de Productos Químicos (CMC), Etiquetado Regulatorio y Servicios de Material de Diseño Regulatorio. Se espera que el segmento de Redacción Médica y Científica alcance una mayor cuota de mercado en 2021, y se prevé que el segmento de Farmacovigilancia registre una mayor tasa de crecimiento anual compuesta (TCAC) durante el período de pronóstico.
Información basada en el usuario final
Según el usuario final, el mercado de externalización de asuntos regulatorios de salud se segmenta en empresas farmacéuticas, empresas de biotecnología y empresas de dispositivos médicos. El segmento de empresas farmacéuticas representaría una mayor cuota de mercado en 2021. Se estima que el mercado de este segmento crecerá a una tasa de crecimiento anual compuesta (TCAC) más alta entre 2021 y 2028.
Las empresas que operan en el mercado de subcontratación de asuntos regulatorios de atención médica adoptan la estrategia de innovación de productos para satisfacer las cambiantes demandas de los clientes en todo el mundo, lo que también les permite mantener su marca en el mercado global.
Perspectivas regionales del mercado de subcontratación de asuntos regulatorios de atención médica
Los analistas de The Insight Partners han explicado detalladamente las tendencias regionales y los factores que influyen en el mercado de externalización de asuntos regulatorios de salud durante el período de pronóstico. Esta sección también analiza los segmentos y la geografía del mercado de externalización de asuntos regulatorios de salud en América del Norte, Europa, Asia Pacífico, Oriente Medio y África, y América del Sur y Central.
Alcance del informe de mercado de subcontratación de asuntos regulatorios de atención médica
| Atributo del informe | Detalles |
|---|---|
| Tamaño del mercado en 2021 | US$ 7.27 mil millones |
| Tamaño del mercado en 2028 | 15 mil millones de dólares estadounidenses |
| CAGR global (2021-2028) | 10,9% |
| Datos históricos | 2019-2020 |
| Período de pronóstico | 2022-2028 |
| Segmentos cubiertos |
Por tipo de servicio
|
| Regiones y países cubiertos |
América del norte
|
| Líderes del mercado y perfiles de empresas clave |
|
Densidad de actores del mercado de externalización de asuntos regulatorios de atención médica: comprensión de su impacto en la dinámica empresarial
El mercado de externalización de asuntos regulatorios en el sector salud está creciendo rápidamente, impulsado por la creciente demanda de los usuarios finales debido a factores como la evolución de las preferencias de los consumidores, los avances tecnológicos y un mayor conocimiento de los beneficios del producto. A medida que aumenta la demanda, las empresas amplían su oferta, innovan para satisfacer las necesidades de los consumidores y aprovechan las tendencias emergentes, lo que impulsa aún más el crecimiento del mercado.
- Obtenga una descripción general de los principales actores clave del mercado de subcontratación de asuntos regulatorios de atención médica
Mercado de subcontratación de asuntos regulatorios de atención médica: por tipo de servicio
- Desarrollo de estrategia regulatoria y científica.
- Redacción médica y científica
- eCTD y presentaciones electrónicas
- Servicios de gestion de datos
- Servicios de gestión del ciclo de vida.
- Farmacovigilancia
- Servicios de fabricación y control de productos químicos (CMC)
- Etiquetado reglamentario
- Servicios de material gráfico regulatorio
Mercado de subcontratación de asuntos regulatorios de atención médica: por usuario final
- Compañías farmacéuticas
- Empresas de biotecnología
-
Empresas de dispositivos médicos
- Software de dispositivos médicos (SaMD)
- Materiales y biomateriales para dispositivos médicos.
- Biomarcadores de dispositivos médicos y diagnóstico in vitro (IVD)
- Dispositivo médico basado en sustancias
- Dispositivo médico de producto combinado (DDC)
Mercado de subcontratación de asuntos regulatorios de atención médica por geografía
-
América del Norte
- A NOSOTROS
- Canadá
- México
-
Europa
- Francia
- Alemania
- Italia
- Reino Unido
- España
- Resto de Europa
-
Asia Pacífico (APAC)
- Porcelana
- India
- Corea del Sur
- Japón
- Australia
- Resto de Asia Pacífico
-
Oriente Medio y África (MEA)
- Sudáfrica
- Arabia Saudita
- Emiratos Árabes Unidos
- Resto de Oriente Medio y África
-
América del Sur y Central (SCAM)
- Brasil
- Argentina
- Resto de América del Sur y Central
Perfiles de empresas
- KLIFO
- Grupo ProPharma
- Arriello Irlanda Ltd.
- DRA CONSULTING OY
- Asphalion SL
- Corporación Internacional Parexel
- IQVIA Inc.
- Pharmalex GmbH
- Grupo ProductLife
- Voisin Consulting Ciencias de la Vida (VCLS)
- Consultoría de soporte científico de contratos de Azierta
- Análisis histórico (2 años), año base, pronóstico (7 años) con CAGR
- Análisis PEST y FODA
- Tamaño del mercado, valor/volumen: global, regional y nacional
- Industria y panorama competitivo
- Conjunto de datos de Excel
Informes recientes
Informes relacionados
Testimonios
Razón para comprar
- Toma de decisiones informada
- Comprensión de la dinámica del mercado
- Análisis competitivo
- Información sobre clientes
- Pronósticos del mercado
- Mitigación de riesgos
- Planificación estratégica
- Justificación de la inversión
- Identificación de mercados emergentes
- Mejora de las estrategias de marketing
- Impulso de la eficiencia operativa
- Alineación con las tendencias regulatorias






















 Obtenga una muestra gratuita para - Mercado de subcontratación de asuntos regulatorios de atención médica
Obtenga una muestra gratuita para - Mercado de subcontratación de asuntos regulatorios de atención médica