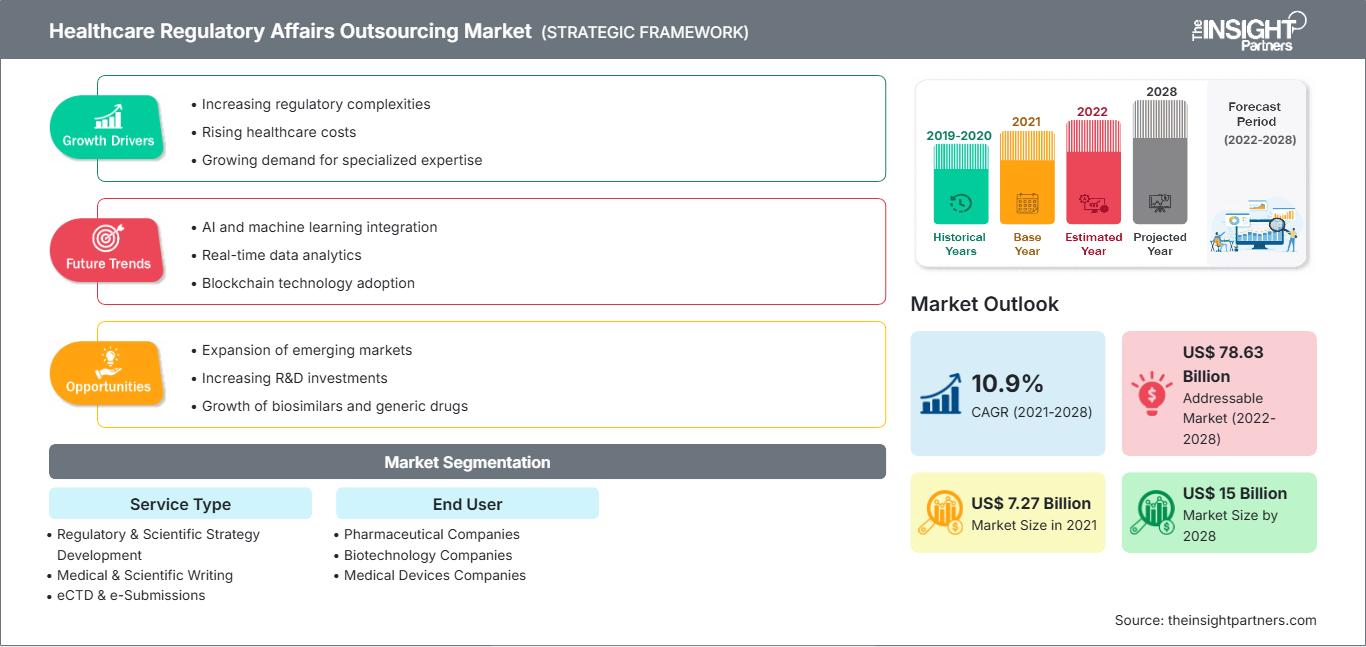
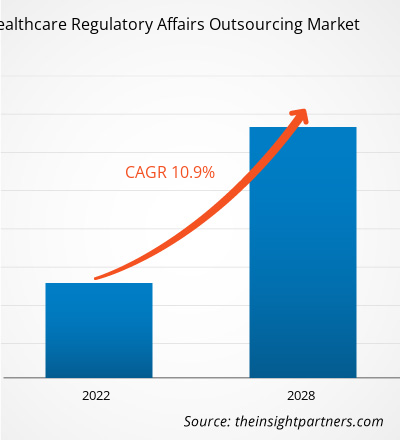
[調査レポート]ヘルスケアの薬事アウトソーシング市場は、2021年の72億7,473万米ドルから2028年には149億9,635万米ドルに達すると予測されており、2021年から2028年にかけて10.9%のCAGRで成長すると見込まれています。
ヘルスケア企業に対する規制圧力の高まりと、新製品の迅速な承認に対する需要の高まり。しかし、熟練した専門家の不足が、ヘルスケアの薬事アウトソーシング市場の成長を抑制しています。薬事アウトソーシングは、製薬、バイオテクノロジー、医療機器製造業界に提供されるサービスです。薬事アウトソーシングサービスは、迅速な規制承認の達成に役立ちます。薬事アウトソーシング業界は、新製品の承認取得、臨床試験実施プロトコルの準備、報告書の発行などを支援しています。薬事コンサルティング、薬事文書のメディカルライティングと発行、臨床試験申請、薬事コンサルティングと法的代理、特許申請、製品登録、臨床試験申請などのさまざまなサービスの需要の増加により、ヘルスケア薬事アウトソーシングビジネスの採用が急増しています。
要件に合わせてレポートをカスタマイズ
レポートの一部、国レベルの分析、Excelデータパックなどを含め、スタートアップ&大学向けに特別オファーや割引もご利用いただけます(無償)
ヘルスケア規制業務アウトソーシング市場: 戦略的洞察
-
このレポートの主要な市場動向を入手してください。この無料サンプルには、市場動向から見積もりや予測に至るまでのデータ分析が含まれます。
市場インサイト
ヘルスケア企業への規制圧力の高まり ヘルスケア規制関連業務アウトソーシング市場の成長
従来の医薬品開発アプローチの継続的なアップグレードと進歩は、ヘルスケア分野に大きな課題をもたらしています。製薬会社と医療関係者は、処方薬のコスト削減という大きなプレッシャーにさらされている一方で、運用コストは急騰しています。規制要件の複雑さ、ブロックバスター医薬品の特許切れによる収益の減少、そして政府や医療保険会社からの医療費削減圧力は、ヘルスケア業界にさらなる課題をもたらしています。こうした困難に直面し、製薬会社は自社のリソースと外部の専門家が提供する専門知識を活用する必要性を認識しています。多くのハイエンドな規制関連コンサルティング会社は、製品ライフサイクル全体にわたる専門知識を提供しています。規制関連業務のアウトソーシングは、スポンサーに経験の蓄積、コストの最適化、生産性の向上をもたらす可能性があります。規制関連アウトソーシング会社は、規制要件をより適切に評価し、最適なソリューションを選択する上で有利な立場にあります。彼らは、規制関連文書の発行システムの導入、運用、維持に関する知識に精通しています。大手製薬企業やバイオテクノロジー企業の多くは、規制および医薬品安全性監視サービスも提供できるコンサルティング企業を求めています。
規制申請の複雑化に伴い、CROの専門知識に対する需要が高まっています。製品開発の初期段階で、製品固有の規制に関するアドバイスと戦略、そして医療規制コンプライアンス対策を計画することは、製品の規制承認にとって極めて重要です。開発初期段階でコンプライアンスに対処しないと、不適切な文書の提出、製造上の見落とし、規制研究の省略、その他の規制要件の不適合により、承認プロセスが遅延することがよくあります。ヘルスケア企業は現在、生産性と業務効率を向上させるために、コアコンピテンシーに注力し、非コア機能をアウトソーシングしています。一般的に、アジア太平洋地域や中東アフリカなどの新興市場で活動するCROに規制機能をアウトソーシングすることで、運用コストを削減し、研究開発活動や既存製品の開発といったコア機能への注力を強化することができます。
サービスタイプに基づく洞察
サービスタイプに基づいて、ヘルスケア規制業務アウトソーシング市場は、規制および科学戦略開発、医療および科学ライティング、eCTDおよび電子提出、データ管理サービス、ライフサイクル管理サービス、医薬品安全性監視、化学製造および管理(CMC)サービス、規制ラベリング、規制アートワークサービスに分類されています。医療および科学ライティングセグメントは2021年に大きな市場シェアを占めると予想されており、医薬品安全性監視セグメントは予測期間中にさらに高いCAGRを記録すると予想されています。
エンドユーザーに基づく洞察
エンドユーザーに基づいて、ヘルスケア規制業務アウトソーシング市場は、製薬会社、バイオテクノロジー会社、医療機器会社に分類されています。製薬会社セグメントは2021年に大きな市場シェアを占めるでしょう。製薬会社セグメントの市場は、2021年から2028年にかけて高いCAGRで成長すると予測されています。
ヘルスケア規制業務アウトソーシング市場で事業を展開している企業は、世界中で進化する顧客の需要を満たすために製品イノベーション戦略を採用しており、これによりグローバル市場でブランド名を維持することも可能になっています。
ヘルスケア規制業務アウトソーシング市場
予測期間を通じてヘルスケア規制業務アウトソーシング市場に影響を与える地域的な動向と要因については、The Insight Partnersのアナリストが詳細に解説しています。このセクションでは、北米、ヨーロッパ、アジア太平洋、中東・アフリカ、中南米におけるヘルスケア規制業務アウトソーシング市場のセグメントと地域についても解説します。
ヘルスケア規制業務アウトソーシング市場レポートの範囲
| レポート属性 | 詳細 |
|---|---|
| の市場規模 2021 | US$ 7.27 Billion |
| 市場規模別 2028 | US$ 15 Billion |
| 世界的なCAGR (2021 - 2028) | 10.9% |
| 過去データ | 2019-2020 |
| 予測期間 | 2022-2028 |
| 対象セグメント |
By サービスタイプ
|
| 対象地域と国 |
北米
|
| 市場リーダーと主要企業の概要 |
|
ヘルスケア規制業務アウトソーシング市場におけるプレーヤーの密度:ビジネスダイナミクスへの影響を理解する
ヘルスケア規制業務アウトソーシング市場は、消費者の嗜好の変化、技術の進歩、製品メリットへの認知度の向上といった要因によるエンドユーザーの需要増加に牽引され、急速に成長しています。需要の増加に伴い、企業はサービスを拡大し、消費者ニーズを満たすためのイノベーションを推進し、新たなトレンドを活用しており、これが市場の成長をさらに加速させています。
- 入手 ヘルスケア規制業務アウトソーシング市場 主要プレーヤーの概要
ヘルスケア規制業務アウトソーシング市場 - サービスタイプ別
- 規制および科学戦略開発
- 医学および科学ライティング
- eCTD および電子提出
- データ管理サービス
- ライフサイクル管理サービス
- ファーマコビジランス
- 化学製造および管理 (CMC) サービス
- 規制ラベリング
- 規制アートワーク サービス
ヘルスケア規制業務アウトソーシング市場 - エンドユーザー別
- 製薬会社
- バイオテクノロジー会社
- 医療機器会社
- 医療機器ソフトウェア (SaMD)
- 医療機器材料およびバイオマテリアル
- 医療機器バイオマーカーおよび体外診断(IVD)
- 医療機器物質ベース
- 医療機器複合製品(DDC)
ヘルスケア規制業務アウトソーシング市場 –地理
- 北アメリカ
- 米国
- カナダ
- メキシコ
- ヨーロッパ
- フランス
- ドイツ
- イタリア
- 英国
- スペイン
- その他の地域ヨーロッパ
- アジア太平洋 (APAC)
- 中国
- インド
- 韓国
- 日本
- オーストラリア
- アジア太平洋のその他の地域
- 中東および日本アフリカ (MEA)
- 南アフリカ
- サウジアラビア
- UAE
- 中東のその他の地域およびその他の地域アフリカ
- 南部およびアフリカ中央アメリカ(SCAM)
- ブラジル
- アルゼンチン
- その他の中南米
企業プロファイル
- KLIFO
- ProPharma Group
- Arriello Ireland Ltd.
- DRA CONSULTING OY
- Asphalion SL
- Parexel International Corporation
- IQVIA Inc.
- Pharmalex Gmbh
- ProductLife Group
- Voisin Consulting Life Sciences (VCLS)
- Azierta Contract Science Support Consulting
- 過去2年間の分析、基準年、CAGRによる予測(7年間)
- PEST分析とSWOT分析
- 市場規模価値/数量 - 世界、地域、国
- 業界と競争環境
- Excel データセット
最新レポート
関連レポート
お客様の声
購入理由
- 情報に基づいた意思決定
- 市場動向の理解
- 競合分析
- 顧客インサイト
- 市場予測
- リスク軽減
- 戦略計画
- 投資の正当性
- 新興市場の特定
- マーケティング戦略の強化
- 業務効率の向上
- 規制動向への対応






















 無料サンプルを入手 - ヘルスケア規制業務アウトソーシング市場
無料サンプルを入手 - ヘルスケア規制業務アウトソーシング市場