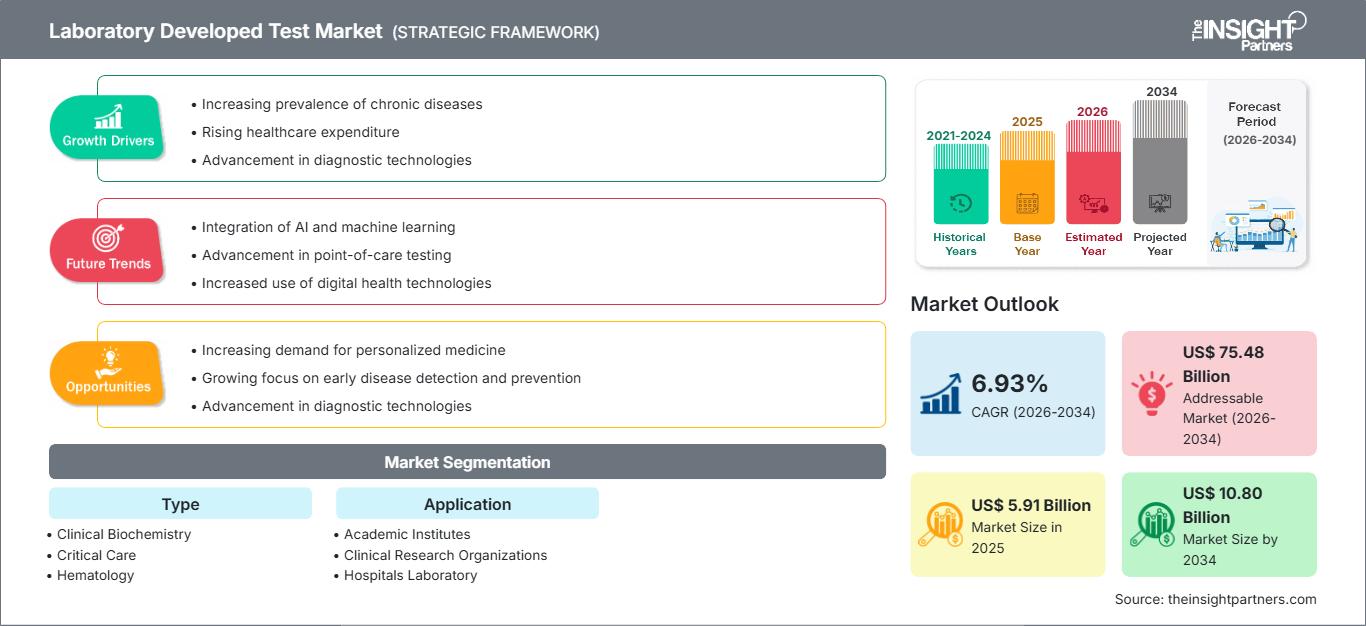
Si prevede che il mercato dei test sviluppati in laboratorio (LDT) raggiungerà i 10,80 miliardi di dollari entro il 2034, rispetto ai 5,91 miliardi di dollari del 2025. Si prevede che il mercato registrerà un CAGR del 6,93% nel periodo 2026-2034.
Analisi di mercato dei test sviluppati in laboratorio
Le previsioni di mercato per i test sviluppati in laboratorio indicano una crescita costante, trainata dalla crescente domanda di medicina personalizzata, dall'aumento dell'incidenza di malattie croniche e rare, dai rapidi progressi nelle tecnologie di test genetici e molecolari e dal ruolo crescente dei laboratori diagnostici nell'assistenza sanitaria di precisione. I test LDT offrono velocità, flessibilità e capacità diagnostiche personalizzabili, consentendo ai laboratori di sviluppare test specializzati laddove potrebbero non esistere test approvati dalla FDA o disponibili in commercio.
L'espansione del mercato è supportata anche dai miglioramenti nel sequenziamento di nuova generazione (NGS), nell'automazione della diagnostica in vitro (IVD) e nelle piattaforme bioinformatiche che consentono ai laboratori di progettare, convalidare e implementare test diagnostici sofisticati. Inoltre, l'evoluzione dei quadri normativi, soprattutto negli Stati Uniti e in Europa, sta spingendo i laboratori ad adottare flussi di lavoro di sviluppo conformi e a investire in sistemi di gestione della qualità.
Panoramica del mercato dei test sviluppati in laboratorio
I test sviluppati in laboratorio (LDT) sono test diagnostici progettati, realizzati e utilizzati internamente da un singolo laboratorio clinico. Questi test sono essenziali per rispondere a esigenze diagnostiche insoddisfatte in aree in cui non esistono test disponibili in commercio o in cui è richiesta una rapida personalizzazione.
Gli LDT supportano il processo decisionale clinico in diverse specialità, tra cui oncologia, malattie infettive, cardiologia, malattie genetiche e salute riproduttiva. Consentono ai laboratori di fornire risultati diagnostici tempestivi, precisi e specifici per il paziente, un requisito sempre più importante nella medicina personalizzata. Consentendo ai laboratori di innovare e rispondere rapidamente alle malattie emergenti, gli LDT stanno diventando parte integrante dei moderni ecosistemi diagnostici.
Personalizza questo report in base alle tue esigenze
Ottieni la PERSONALIZZAZIONE GRATUITAMercato dei test sviluppati in laboratorio: approfondimenti strategici
-
Scopri le principali tendenze di mercato di questo rapporto.Questo campione GRATUITO includerà analisi dei dati, che spaziano dalle tendenze di mercato alle stime e alle previsioni.
Driver e opportunità del mercato dei test sviluppati in laboratorio
Fattori trainanti del mercato:
- Crescente domanda di medicina personalizzata e di precisione: il passaggio globale alla medicina personalizzata è una delle principali forze trainanti dell'adozione della LDT. I medici si affidano sempre più a strumenti diagnostici molecolari e genetici per personalizzare i piani di trattamento. Le LDT offrono la flessibilità necessaria per progettare test specializzati per un'assistenza personalizzata.
- Crescente prevalenza di malattie croniche e rare: la crescente incidenza di tumori, malattie ereditarie, malattie autoimmuni e malattie infettive richiede piattaforme diagnostiche avanzate. I test diagnostici a bassa intensità consentono la diagnosi precoce, la selezione della terapia e il monitoraggio, alimentando la domanda del mercato.
- Progressi in genomica, NGS e bioinformatica: i progressi tecnologici, in particolare nel sequenziamento, nella PCR multiplex e nella proteomica, consentono ai laboratori di creare test ad alta complessità con un'accuratezza superiore. Queste innovazioni accelerano i cicli di sviluppo dei test LDT.
- Un panorama normativo in evoluzione incoraggia il miglioramento della qualità: quadri normativi come CLIA negli Stati Uniti e IVDR in Europa stanno spingendo i laboratori a standardizzare i processi di sviluppo e a migliorare la qualità dei test. Questa attenzione alla conformità sta aumentando gli investimenti nello sviluppo di test LDT a livello globale.
Opportunità di mercato:
- Espansione nei mercati emergenti con infrastrutture sanitarie in rapida evoluzione: Paesi come Cina, India, Brasile e la regione del Golfo stanno investendo massicciamente nelle capacità diagnostiche. Ciò crea opportunità per l'adozione avanzata di LDT in oncologia, genetica e malattie infettive.
- Integrazione di intelligenza artificiale/apprendimento automatico per l'interpretazione dei risultati e l'automazione del flusso di lavoro: l'analisi basata sull'intelligenza artificiale consente un'interpretazione più rapida e accurata dei dati dei test genomici e molecolari. Questo trasforma le capacità di LDT supportando analisi ad alta produttività e ad alta intensità di dati.
- Utilizzo crescente di test LDT per la sorveglianza delle malattie infettive e la risposta alle epidemie: durante le emergenze sanitarie, come le epidemie virali, i test LDT possono essere sviluppati e implementati rapidamente, fornendo soluzioni diagnostiche più rapide rispetto ai test disponibili in commercio.
- Opportunità per i laboratori certificati CLIA ad alta complessità di ampliare i menu dei test: le grandi reti di laboratori diagnostici possono ampliare la propria offerta sviluppando pannelli LDT proprietari, migliorando la competitività e le opportunità di guadagno.
Analisi della segmentazione del rapporto di mercato dei test sviluppati in laboratorio
La quota di mercato dei test sviluppati in laboratorio viene analizzata in vari segmenti per chiarire la struttura, il potenziale di crescita e le tendenze emergenti.
Per tipo:
- Biochimica clinica
- Terapia intensiva
- Ematologia
- Microbiologia
- Diagnostica molecolare
- Immunologia
Per applicazione:
- Istituti accademici
- Organizzazioni di ricerca clinica
- Laboratorio Ospedaliero
- Centri diagnostici specialistici
Per geografia:
- America del Nord
- Europa
- Asia Pacifico
- America meridionale e centrale
- Medio Oriente e Africa
Approfondimenti regionali sul mercato dei test sviluppati in laboratorio
Le tendenze e i fattori regionali che hanno influenzato il mercato dei test di laboratorio durante il periodo di previsione sono stati ampiamente spiegati dagli analisti di The Insight Partners. Questa sezione illustra anche i segmenti e la distribuzione geografica del mercato dei test di laboratorio in Nord America, Europa, Asia-Pacifico, Medio Oriente e Africa, America Meridionale e Centrale.
Ambito del rapporto di mercato sui test sviluppati in laboratorio
| Attributo del report | Dettagli |
|---|---|
| Dimensioni del mercato nel 2025 | 5,91 miliardi di dollari USA |
| Dimensioni del mercato entro il 2034 | 10,80 miliardi di dollari USA |
| CAGR globale (2026 - 2034) | 6,93% |
| Dati storici | 2021-2024 |
| Periodo di previsione | 2026-2034 |
| Segmenti coperti |
Per tipo
|
| Regioni e paesi coperti |
America del Nord
|
| Leader di mercato e profili aziendali chiave |
|
Densità dei test di mercato sviluppati in laboratorio: comprendere il suo impatto sulle dinamiche aziendali
Il mercato dei test sviluppati in laboratorio è in rapida crescita, trainato dalla crescente domanda degli utenti finali, dovuta a fattori quali l'evoluzione delle preferenze dei consumatori, i progressi tecnologici e una maggiore consapevolezza dei benefici dei prodotti. Con l'aumento della domanda, le aziende stanno ampliando la propria offerta, innovando per soddisfare le esigenze dei consumatori e sfruttando le tendenze emergenti, alimentando ulteriormente la crescita del mercato.
- Ottieni una panoramica dei principali attori del mercato dei test sviluppati in laboratorio
Analisi della quota di mercato dei test sviluppati in laboratorio per area geografica
America del Nord
- Quota di mercato: la quota più ampia a livello mondiale grazie all'infrastruttura avanzata di diagnostica molecolare e all'elevata adozione della medicina di precisione.
-
Fattori chiave:
- Diffuso ecosistema di laboratori certificati CLIA
- Adozione precoce di NGS e test ad alta complessità
- Forte attenzione all'oncologia e alla diagnostica genetica
- Tendenze: crescente adozione di flussi di lavoro LDT basati sull'intelligenza artificiale ed espansione dei test decentralizzati.
Europa
- Quota di mercato: quota significativa determinata da rigorosi standard di qualità e dall'implementazione dell'IVDR.
-
Fattori chiave:
- Richiesta di analisi di laboratorio conformi e di alta qualità
- Programmi nazionali di genomica
- Crescita nella diagnostica delle malattie rare
- Tendenze: crescente adozione di piattaforme diagnostiche interoperabili per uno scambio continuo di dati dei pazienti.
Asia Pacifico
- Quota di mercato: regione in più rapida crescita.
-
Fattori chiave:
- Iniziative di genomica e medicina di precisione sostenute dal governo
- Crescente prevalenza di cancro, malattie genetiche e malattie infettive
- Rapida espansione delle infrastrutture
- Tendenze: diagnostica molecolare basata sull'intelligenza artificiale e soluzioni di test localizzati per popolazioni diverse.
America meridionale e centrale
- Quota di mercato: in crescita con la modernizzazione dei sistemi di laboratorio.
-
Fattori chiave:
- Partenariati pubblico-privati
- Necessità di soluzioni diagnostiche convenienti.
- Espansione dei laboratori diagnostici privati
- Tendenze: software di interpretazione basato sul cloud e flussi di lavoro LDT convenienti.
Medio Oriente e Africa
- Quota di mercato: in via di sviluppo ma in rapido miglioramento.
-
Fattori chiave:
- Strategie nazionali per la medicina di precisione
- Crescita delle capacità avanzate di oncologia e test genetici
- Investimenti in infrastrutture diagnostiche
- Tendenze: integrazione degli LDT in piattaforme più ampie di sorveglianza sanitaria digitale e di salute pubblica.
Densità dei test sviluppati in laboratorio: impatto sulle dinamiche aziendali
Le strategie competitive si concentrano su:
- Espansione dei portafogli proprietari di test oncologici e genetici
- Integrazione di NGS, intelligenza artificiale e bioinformatica nello sviluppo di LDT
- Rapido sviluppo di test per le malattie infettive per le esigenze di salute pubblica
- Partnership tra reti di laboratori e fornitori di tecnologia
Opportunità e mosse strategiche
- Investimenti in piattaforme di sequenziamento automatizzato e test molecolari
- Collaborazione con aziende biotecnologiche per lo sviluppo congiunto di pannelli diagnostici avanzati
- Espansione nelle regioni emergenti con crescente domanda diagnostica
Le principali aziende che operano nel mercato dei test sviluppati in laboratorio
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD.
- QIAGEN
- Illumina, Inc.
- Eurofins Scientific
- Biodesix
- Adattivo
- Biotecnologie Bioteranostica
- Rosetta Genomics Ltd.
Altre aziende analizzate nel corso della ricerca:
- PerkinElmer Inc.
- Thermo Fisher Scientific Inc.
- Roche Diagnostics
- GeneDx
- 10x Genomica
- Natera Inc.
- Società di scienze esatte
- Agilent Technologies
- Helix OpCo LLC
Notizie di mercato e sviluppi recenti sui test sviluppati in laboratorio
- Quest Diagnostics ha ampliato il suo portafoglio oncologico lanciando nuovi pannelli LDT basati su NGS per tumori solidi e neoplasie ematologiche a supporto delle iniziative di oncologia di precisione.
- LabCorp ha annunciato la convalida di diversi test LDT di patologia assistiti dall'intelligenza artificiale, migliorando l'accuratezza diagnostica e i tempi di risposta nella rilevazione e classificazione del cancro.
- I laboratori della Mayo Clinic hanno introdotto nuovi pannelli LDT per malattie rare, consentendo una diagnosi rapida attraverso metodi di sequenziamento ad alta complessità.
- Fulgent Genetics ha ampliato l'offerta di test LDT per le malattie infettive, includendo pannelli respiratori rapidi e test virali, rafforzando le sue capacità di test per la salute pubblica.
Copertura e risultati del rapporto di mercato sui test sviluppati in laboratorio
Il rapporto "Dimensioni e previsioni del mercato dei test sviluppati in laboratorio (2021-2034)" fornisce un'analisi dettagliata che comprende:
- Dimensioni e previsioni del mercato LDT (a livello globale, regionale, nazionale)
- Tendenze, fattori trainanti, vincoli e opportunità del mercato
- Analisi PEST e SWOT dettagliate
- Panorama competitivo, analisi della concentrazione e posizionamento di mercato
- Panorama normativo (CLIA, IVDR, quadri globali)
- Profili aziendali, portafogli prodotti e sviluppi strategici
- Analisi storica (2 anni), anno base, previsione (7 anni) con CAGR
- Analisi PEST e SWOT
- Valore/volume delle dimensioni del mercato - Globale, Regionale, Nazionale
- Industria e panorama competitivo
- Set di dati Excel
Report recenti
Testimonianze
Motivo dell'acquisto
- Processo decisionale informato
- Comprensione delle dinamiche di mercato
- Analisi competitiva
- Analisi dei clienti
- Previsioni di mercato
- Mitigazione del rischio
- Pianificazione strategica
- Giustificazione degli investimenti
- Identificazione dei mercati emergenti
- Miglioramento delle strategie di marketing
- Aumento dell'efficienza operativa
- Allineamento alle tendenze normative






















 Ottieni un campione gratuito per - Mercato dei test sviluppati in laboratorio
Ottieni un campione gratuito per - Mercato dei test sviluppati in laboratorio