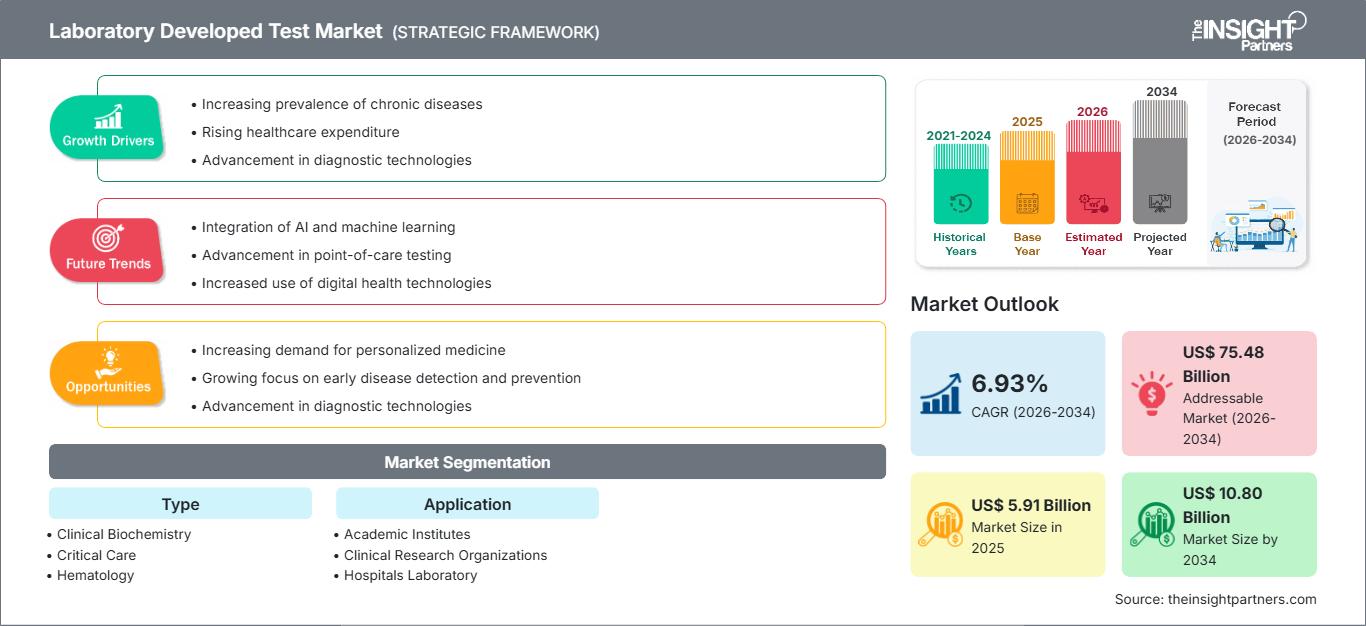
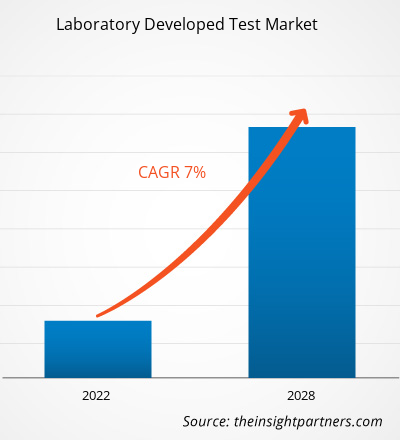
臨床検査(LDT)市場規模は、2025年の59億1,000万米ドルから2034年には108億米ドルに達すると予想されています。市場は2026年から2034年の間に6.93%のCAGRを記録すると予想されています。
ラボ開発テスト市場分析
臨床検査(LDT)市場の予測は、個別化医療への需要の高まり、慢性疾患および希少疾患の負担増大、遺伝子・分子検査技術の急速な進歩、そして精密医療における診断ラボの役割拡大を背景に、着実な成長を示しています。LDTは、迅速性、柔軟性、そしてカスタマイズ可能な診断機能を備えており、FDA承認済みまたは市販の検査が存在しない場合でも、ラボが専門的な検査を開発することを可能にします。
市場の拡大は、次世代シーケンシング(NGS)、体外診断(IVD)自動化、そしてバイオインフォマティクス・プラットフォームの進歩によって支えられており、これらのプラットフォームは、検査室が高度な診断アッセイを設計、検証、そして展開することを可能にします。さらに、特に米国と欧州における規制枠組みの進化は、検査室が規制に準拠した開発ワークフローを導入し、品質管理システムへの投資を促しています。
臨床検査市場の概要
臨床検査開発(LDT)とは、単一の臨床検査室で設計、製造、使用される社内診断検査です。これらの検査は、市販の検査が存在しない、あるいは迅速なカスタマイズが求められる分野における、満たされていない診断ニーズへの対応に不可欠です。
LDTは、腫瘍学、感染症、心臓病学、遺伝性疾患、生殖医療など、複数の専門分野における臨床意思決定を支援します。LDTにより、検査室はタイムリーで正確、かつ患者固有の診断結果を提供することが可能になり、これは個別化医療においてますます重要な要件となっています。検査室が革新を起こし、新興疾患に迅速に対応することを可能にするLDTは、現代の診断エコシステムに不可欠な存在になりつつあります。
要件に合わせてレポートをカスタマイズ
無料カスタマイズ臨床検査市場:戦略的洞察
-
このレポートの主要な市場動向を入手してください。この無料サンプルには、市場動向から見積もりや予測に至るまでのデータ分析が含まれます。
ラボ開発テスト市場の推進要因と機会
市場の推進要因:
- 個別化医療と精密医療への需要の高まり:個別化医療への世界的な移行は、LDT導入の大きな推進力となっています。臨床医は、治療計画をカスタマイズするために、分子・遺伝子診断ツールへの依存度が高まっています。LDTは、個別化されたケアのための専門的な検査を設計するために必要な柔軟性を提供します。
- 慢性疾患および希少疾患の罹患率の上昇:がん、遺伝性疾患、自己免疫疾患、感染症の発生率の上昇により、高度な診断プラットフォームが求められています。LDTは早期発見、治療法の選択、モニタリングを可能にし、市場の需要を高めています。
- ゲノミクス、NGS、バイオインフォマティクスの進歩:特にシーケンシング、マルチプレックスPCR、プロテオミクスにおける技術の進歩により、検査室は高度な検査を優れた精度で作成できるようになりました。これらのイノベーションは、LDTの開発サイクルを加速させます。
- 品質向上を促す規制環境の進化:米国のCLIAや欧州のIVDRといった規制枠組みは、検査室に開発プロセスの標準化と検査品質の向上を促しています。こうしたコンプライアンスへの重点的な取り組みは、LDT開発への世界的な投資増加につながっています。
市場機会:
- 急速に進化する医療インフラを持つ新興市場への進出:中国、インド、ブラジル、湾岸地域などの国々は、診断能力に多額の投資を行っています。これにより、腫瘍学、遺伝学、感染症分野における高度なLDT導入の機会が生まれます。
- 結果解釈とワークフロー自動化のためのAI/MLの統合:AI主導の分析により、ゲノムおよび分子検査データのより迅速かつ正確な解釈が可能になります。これにより、高スループットでデータ集約的な分析をサポートし、LDT機能を変革します。
- 感染症の監視と発生時の対応における LDT の使用の増加: ウイルスの発生などの健康上の緊急事態が発生すると、LDT を迅速に開発して展開することができ、市販の検査よりも迅速に診断ソリューションを提供できます。
- 高度に複雑な CLIA 認定ラボが検査メニューを拡張する機会: 大規模な診断ラボ ネットワークは、独自の LDT パネルを開発することで提供内容を拡張し、競争力と収益機会を高めることができます。
臨床検査市場レポート:セグメンテーション分析
研究室で開発されたテストの市場シェアは、構造、成長の可能性、新たな傾向を明確にするために、さまざまなセグメントにわたって分析されています。
タイプ別:
- 臨床生化学
- 集中治療
- 血液学
- 微生物学
- 分子診断
- 免疫学
用途別:
- 学術機関
- 臨床研究機関
- 病院の検査室
- 専門診断センター
地理別:
- 北米
- ヨーロッパ
- アジア太平洋
- 南米と中央アメリカ
- 中東・アフリカ
臨床検査市場における地域分析
予測期間全体を通して、臨床検査市場に影響を与える地域的な傾向と要因は、The Insight Partnersのアナリストによって徹底的に説明されています。このセクションでは、臨床検査市場のセグメントと地域についても、北米、ヨーロッパ、アジア太平洋、中東・アフリカ、中南米に分けて解説しています。
臨床検査市場レポートの範囲
| レポート属性 | 詳細 |
|---|---|
| 2025年の市場規模 | 59億1000万米ドル |
| 2034年までの市場規模 | 108億米ドル |
| 世界のCAGR(2026年~2034年) | 6.93% |
| 履歴データ | 2021-2024 |
| 予測期間 | 2026~2034年 |
| 対象セグメント |
タイプ別
|
| 対象地域と国 |
北米
|
| 市場リーダーと主要企業の概要 |
|
臨床検査市場におけるプレーヤーの密度:ビジネスダイナミクスへの影響を理解する
臨床検査市場は、消費者の嗜好の変化、技術の進歩、製品の利点に対する認知度の高まりといった要因によるエンドユーザーの需要増加に牽引され、急速に成長しています。需要の増加に伴い、企業は提供内容を拡大し、消費者ニーズを満たすための革新を進め、新たなトレンドを活用しており、これが市場の成長をさらに促進しています。
- ラボ開発テスト市場のトップキープレーヤーの概要を入手
地域別臨床検査市場シェア分析
北米
- 市場シェア: 高度な分子診断インフラストラクチャと高精度医療の採用率の高さにより、世界最大のシェアを獲得しています。
-
主な推進要因:
- 広範囲にわたるCLIA認定ラボエコシステム
- NGSと高複雑性検査の早期導入
- 腫瘍学と遺伝子診断に重点を置いています
- トレンド: AI 対応の LDT ワークフローの採用の増加と分散型テストの拡大。
ヨーロッパ
- 市場シェア: 厳格な品質基準と IVDR の実装により大きなシェアを獲得しました。
-
主な推進要因:
- コンプライアンスに準拠した高品質の検査の需要
- 国立ゲノムプログラム
- 希少疾患診断の成長
- トレンド: シームレスな患者データ交換を実現する相互運用可能な診断プラットフォームの採用が増加しています。
アジア太平洋
- 市場シェア: 最も急速に成長している地域。
-
主な推進要因:
- 政府が支援するゲノミクスと精密医療の取り組み
- がん、遺伝性疾患、感染症の増加
- 急速なインフラ拡張
- トレンド: 多様な集団を対象とした AI 駆動型分子診断とローカライズされたテスト ソリューション。
南米と中央アメリカ
- 市場シェア: 実験システムの近代化とともに成長しています。
-
主な推進要因:
- 官民パートナーシップ
- 費用対効果の高い診断ソリューションの必要性。
- 民間診断研究所の拡大
- トレンド: クラウド対応の通訳ソフトウェアとコスト効率の高い LDT ワークフロー。
中東・アフリカ
- 市場シェア: 発展途上だが急速に向上中。
-
主な推進要因:
- 国家精密医療戦略
- 高度な腫瘍学および遺伝子検査能力の成長
- 診断インフラへの投資
- トレンド: LDT をより広範なデジタルヘルスおよび公衆衛生監視プラットフォームに統合します。
臨床検査市場におけるプレーヤー密度:ビジネスダイナミクスへの影響
競争戦略の焦点は次の通りです。
- 独自の腫瘍学および遺伝子検査ポートフォリオの拡大
- LDT開発へのNGS、AI、バイオインフォマティクスの統合
- 公衆衛生ニーズに応える感染症検査の迅速な開発
- 研究室ネットワークと技術ベンダーとのパートナーシップ
機会と戦略的動き
- 自動シーケンシングおよび分子検査プラットフォームへの投資
- バイオテクノロジー企業との連携による高度な診断パネルの共同開発
- 診断需要の高まりによる新興地域への進出
ラボ開発テスト市場で事業を展開する主要企業
- クエスト・ダイアグノスティクス社
- F. ホフマン・ラ・ロッシュ株式会社
- キアゲン
- イルミナ株式会社
- ユーロフィンサイエンティフィック
- バイオデシックス
- 適応型
- バイオテクノロジー
- ロゼッタジェノミクス株式会社
調査の過程で分析された他の企業:
- パーキンエルマー株式会社
- サーモフィッシャーサイエンティフィック株式会社
- ロシュ・ダイアグノスティックス
- ジーンDx
- 10倍ゲノミクス
- ナテラ株式会社
- エグザクトサイエンシズコーポレーション
- アジレント・テクノロジーズ
- ヘリックス・オプコLLC
臨床検査市場のニュースと最近の動向
- Quest Diagnostics は、精密腫瘍学の取り組みをサポートするために、固形腫瘍および血液悪性腫瘍用の新しい NGS ベースの LDT パネルを発売し、腫瘍学のポートフォリオを拡大しました。
- LabCorp は、がんの検出と分類における診断精度と処理時間を向上させる複数の AI 支援病理学 LDT の検証を発表しました。
- メイヨー クリニック ラボラトリーズは、新しい希少疾患 LDT パネルを導入し、高度に複雑なシーケンス法による迅速な診断を可能にしました。
- フルジェント・ジェネティクスは、迅速な呼吸器およびウイルスパネルを含む感染症LDTの提供を拡大し、公衆衛生検査能力を強化しました。
ラボ開発テスト市場レポートの対象範囲と成果物
「ラボ開発テスト市場の規模と予測(2021〜2034年)」レポートでは、以下の詳細な分析を提供しています。
- LDT市場規模と予測(世界、地域、国レベル)
- 市場の動向、推進要因、制約、機会
- 詳細なPEST分析とSWOT分析
- 競争環境、集中分析、市場ポジショニング
- 規制の状況(CLIA、IVDR、グローバルフレームワーク)
- 企業概要、製品ポートフォリオ、戦略開発
- 過去2年間の分析、基準年、CAGRによる予測(7年間)
- PEST分析とSWOT分析
- 市場規模価値/数量 - 世界、地域、国
- 業界と競争環境
- Excel データセット
最新レポート
お客様の声
購入理由
- 情報に基づいた意思決定
- 市場動向の理解
- 競合分析
- 顧客インサイト
- 市場予測
- リスク軽減
- 戦略計画
- 投資の正当性
- 新興市場の特定
- マーケティング戦略の強化
- 業務効率の向上
- 規制動向への対応






















 無料サンプルを入手 - ラボ開発テスト市場
無料サンプルを入手 - ラボ開発テスト市場