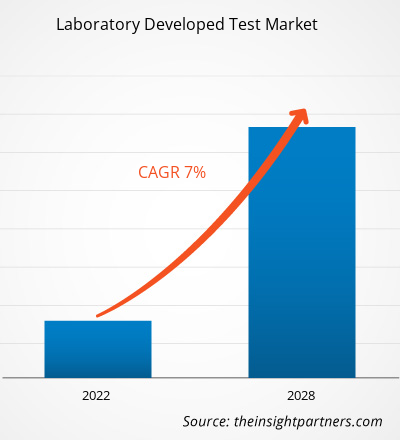
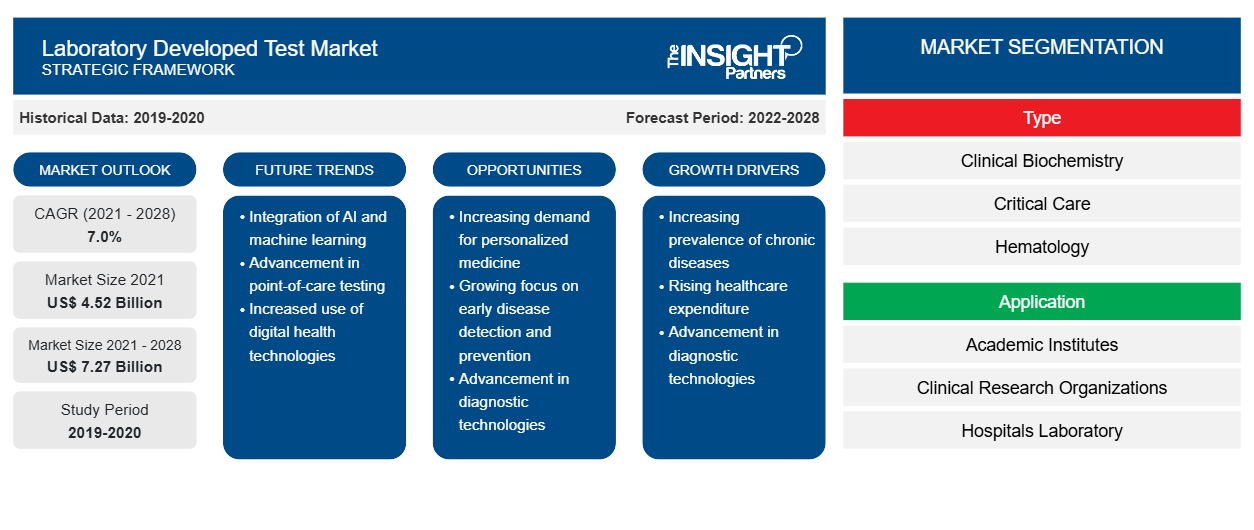
Le marché des tests développés en laboratoire devrait atteindre 7 269,3 millions USD d'ici 2028, contre 4 524,75 millions USD en 2021 ; il devrait croître à un TCAC de 7,0 % au cours de la période 2021-2028.
Un test développé en laboratoire (LDT) est un type de test de diagnostic in vitro conçu et utilisé dans un seul laboratoire. Ces tests peuvent être utilisés pour estimer ou distinguer des analytes tels que des protéines, des biomolécules/composés (glucose, cholestérol, etc.) et de l'ADN extraits d'échantillons prélevés sur des sujets humains. L'expansion des méthodes de diagnostic in vitro automatisées (DIV) pour les laboratoires et les dispensaires afin de rendre des analyses précises et sans erreur alimente la croissance du marché des tests développés en laboratoire.
La croissance du marché des tests développés en laboratoire est principalement attribuée à des facteurs tels que l'augmentation des cas de cancer et de troubles génétiques et le grand nombre de lancements de produits. Cependant, l'évolution du paysage réglementaire freine la croissance du marché. Par exemple, en Europe, la conformité au règlement sur les dispositifs in vitro (IVDR) sera obligatoire pour tous les tests de diagnostic in vitro à partir de mai 2022 ; le règlement vise à garantir l'efficacité clinique et la sécurité des tests médicaux, transformant ainsi l'industrie du diagnostic, ce qui constitue une grande préoccupation pour les acteurs du marché.
Personnalisez ce rapport en fonction de vos besoins
Vous bénéficierez d'une personnalisation gratuite de n'importe quel rapport, y compris de certaines parties de ce rapport, d'une analyse au niveau des pays, d'un pack de données Excel, ainsi que de superbes offres et réductions pour les start-ups et les universités.
-
Obtenez les principales tendances clés du marché de ce rapport.Cet échantillon GRATUIT comprendra une analyse de données, allant des tendances du marché aux estimations et prévisions.
Informations sur le marché
La recherche continue sur les médicaments personnalisés offre des opportunités de croissance aux acteurs du marché des tests développés en laboratoire
Les LDT jouent un rôle essentiel dans le développement de médicaments personnalisés qui sont susceptibles de s'avérer être des moyens prometteurs de lutter contre les maladies grâce à des traitements ou des remèdes efficaces jusqu'ici inexistants. Selon la Personalized Medicine Coalition, les médicaments personnalisés ne représentaient que 5 % des nouvelles entités moléculaires approuvées par la FDA en 2005 ; cependant, en 2016, ce chiffre est passé à plus de 25 %. En outre, 42 % de tous les composés et 73 % des composés oncologiques en cours de développement ont le potentiel de servir de médicaments personnalisés. Les sociétés biopharmaceutiques ont presque doublé leurs investissements en R&D dans les médicaments personnalisés au cours des cinq dernières années, et elles devraient encore augmenter leurs investissements de 33 % au cours des cinq prochaines années. Les chercheurs biopharmaceutiques prévoient également une augmentation de 69 % du développement de médicaments personnalisés au cours des cinq prochaines années. Les tests de laboratoire sont utilisés pour diagnostiquer les maladies et prédire et surveiller la réponse aux médicaments, ainsi que pour obtenir les données informatiques nécessaires aux algorithmes prédictifs complexes. play a vital role in the development of personalized medicines that are likely to prove as promising means of tackling diseases through far eluded effective treatments or cures. As per the Personalized Medicine Coalition, personalized medicines accounted for only 5% of the new FDA-approved molecular entities in 2005; however, in 2016, this number rose to more than 25%. Additionally, 42% of all compounds and 73% of oncology compounds in the pipeline have the potential to serve as personalized medicines. Biopharmaceutical companies have nearly doubled their R&D investments in personalized drugs in the last five years, and they are further expected to increase their investments by 33% in the next five years. Biopharmaceutical researchers also predict a 69% increase in the development of personalized medicines in the next five years. Laboratory tests are used to diagnose illness and predict and monitor drug response as well as to obtain informatics data needed for complex predictive algorithms.
Les médicaments personnalisés sont en train de devenir la marque de fabrique du traitement du cancer. Il s’agit d’une approche en constante évolution qui repose sur la personnalisation des traitements en fonction de la constitution génétique individuelle. En 2019, la FDA a approuvé 12 médicaments personnalisés pour étudier et traiter les causes profondes de la maladie, combinant ainsi la médecine de précision aux soins cliniques. La demande croissante de médecine personnalisée offre des opportunités de croissance importantes pour la croissance des acteurs opérant sur le marché des tests développés en laboratoire.
Informations basées sur les types
Le marché des tests développés en laboratoire, par type, est segmenté en biochimie clinique, soins intensifs, hématologie, microbiologie, diagnostic moléculaire, immunologie et autres. Le segment du diagnostic moléculaire devrait détenir la plus grande part du marché en 2021. Cependant, le segment de l'hématologie devrait enregistrer le TCAC le plus élevé du marché au cours de la période de prévision.CAGR in the market during the forecast period.
Informations basées sur les applications
En termes d'application, le laboratoire a développé des applications de marché de test dans les instituts universitaires, les organismes de recherche clinique, les laboratoires hospitaliers, les centres de diagnostic spécialisés et autres. Le segment des laboratoires hospitaliers devrait représenter la plus grande part de marché en 2021. Cependant, le segment des centres de diagnostic spécialisés devrait enregistrer le TCAC le plus élevé du marché au cours de la période de prévision.CAGR in the market during the forecast period.
Les lancements et les approbations de produits sont des stratégies couramment adoptées par les entreprises pour étendre leur présence mondiale et leurs portefeuilles de produits. De plus, les acteurs du marché test développés en laboratoire se concentrent sur la stratégie de partenariat pour élargir leur clientèle, ce qui leur permet à leur tour de maintenir leur nom de marque à l'échelle mondiale.
Le rapport segmente le marché des tests développés en laboratoire comme suit
Français En fonction du type, le marché des tests développés en laboratoire est segmenté en biochimie clinique, soins intensifs, hématologie, microbiologie, diagnostic moléculaire, immunologie et autres. En fonction de l'application, le marché des tests développés en laboratoire est segmenté en instituts universitaires, organismes de recherche clinique, laboratoires hospitaliers, centres de diagnostic spécialisés et autres. Sur la base de la géographie, le marché des tests développés en laboratoire est segmenté en Amérique du Nord (États-Unis, Canada et Mexique), Europe (Royaume-Uni, Allemagne, France, Italie, Espagne et reste de l'Europe), Asie-Pacifique (Chine, Japon, Inde, Australie, Corée du Sud et reste de l'Asie-Pacifique), Moyen-Orient et Afrique (Émirats arabes unis, Arabie saoudite, Afrique du Sud et reste du Moyen-Orient et de l'Afrique) et Amérique du Sud et centrale (Brésil, Argentine et reste de l'Amérique du Sud et centrale).
Aperçu régional du marché des tests développés en laboratoire
Les tendances et facteurs régionaux influençant le marché des tests développés en laboratoire tout au long de la période de prévision ont été expliqués en détail par les analystes d’Insight Partners. Cette section traite également des segments et de la géographie du marché des tests développés en laboratoire en Amérique du Nord, en Europe, en Asie-Pacifique, au Moyen-Orient et en Afrique, ainsi qu’en Amérique du Sud et en Amérique centrale.
- Obtenez les données régionales spécifiques au marché des tests développés en laboratoire
Portée du rapport sur le marché des tests développés en laboratoire
| Attribut de rapport | Détails |
|---|---|
| Taille du marché en 2021 | 4,52 milliards de dollars américains |
| Taille du marché d'ici 2028 | 7,27 milliards de dollars américains |
| Taux de croissance annuel composé mondial (2021-2028) | 7,0% |
| Données historiques | 2019-2020 |
| Période de prévision | 2022-2028 |
| Segments couverts |
Par type
|
| Régions et pays couverts |
Amérique du Nord
|
| Leaders du marché et profils d'entreprises clés |
|
Densité des acteurs du marché des tests développés en laboratoire : comprendre son impact sur la dynamique des entreprises
Le marché des tests développés en laboratoire connaît une croissance rapide, tirée par la demande croissante des utilisateurs finaux en raison de facteurs tels que l'évolution des préférences des consommateurs, les avancées technologiques et une plus grande sensibilisation aux avantages du produit. À mesure que la demande augmente, les entreprises élargissent leurs offres, innovent pour répondre aux besoins des consommateurs et capitalisent sur les tendances émergentes, ce qui alimente davantage la croissance du marché.
La densité des acteurs du marché fait référence à la répartition des entreprises ou des sociétés opérant sur un marché ou un secteur particulier. Elle indique le nombre de concurrents (acteurs du marché) présents sur un marché donné par rapport à sa taille ou à sa valeur marchande totale.
Les principales entreprises opérant sur le marché des tests développés en laboratoire sont :
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTÉE,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientifique
Avis de non-responsabilité : les sociétés répertoriées ci-dessus ne sont pas classées dans un ordre particulier.
- Obtenez un aperçu des principaux acteurs du marché des tests développés en laboratoire
Profils d'entreprise
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTÉE,
- QIAGEN
- Illumina, Inc.,
- Eurofins Scientifique
- Biodesix
- Biotechnologies adaptatives
- Biothéranostique
- Rosetta Genomics Ltd.,
- Santé de Guardant
- Analyse historique (2 ans), année de base, prévision (7 ans) avec TCAC
- Analyse PEST et SWOT
- Taille du marché Valeur / Volume - Mondial, Régional, Pays
- Industrie et paysage concurrentiel
- Ensemble de données Excel
Rapports récents
Témoignages
Raison d'acheter
- Prise de décision éclairée
- Compréhension de la dynamique du marché
- Analyse concurrentielle
- Connaissances clients
- Prévisions de marché
- Atténuation des risques
- Planification stratégique
- Justification des investissements
- Identification des marchés émergents
- Amélioration des stratégies marketing
- Amélioration de l'efficacité opérationnelle
- Alignement sur les tendances réglementaires






















 Obtenez un échantillon gratuit pour - Marché des tests développés en laboratoire
Obtenez un échantillon gratuit pour - Marché des tests développés en laboratoire