Electronic Trial Master File (eTMF) Market Share and Forecast by 2027
Electronic Trial Master File (eTMF) Market to 2027 - Industry Analysis and Forecasts By Component (Service, Software); Delivery Mode (Cloud-Based eTMF, On-Premise eTMF), End-User (Pharmaceutical and Biotechnology Companies, CROs, Others); and Geography
Historic Data: 2016-2017 | Base Year: 2018 | Forecast Period: 2019-2027- Report Date : Feb 2020
- Report Code : TIPRE00007727
- Category : Technology, Media and Telecommunications
- Status : Published
- Available Report Formats :


- No. of Pages : 145
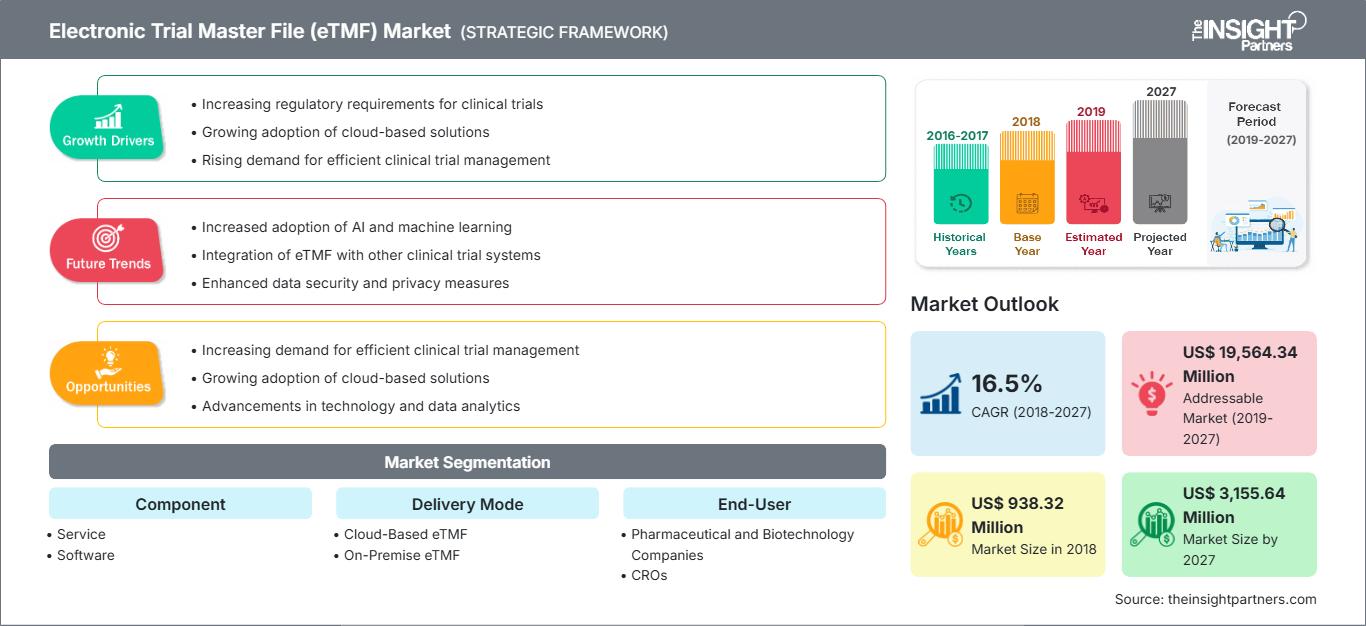
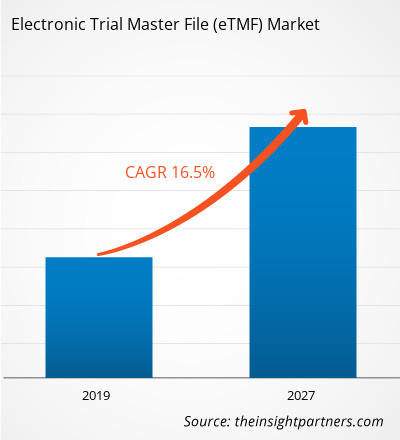
[Research Report] The Electronic Trial Master File in healthcare market was valued at US$ 938.32 million in 2018 and it is projected to reach US$ 3,155.64 million by 2027; it is expected to grow at a CAGR of 16.5% from 2019 to 2027.
Electronic trial master file (eTMF) systems can be defined as an integration of software and hardware components collectively responsible for the optimal management of clinical trial data. These solutions help to streamline the data generated during the course of a clinical trial in an easy-to-store digital format, which can be retrieved by dissimilar users beneficial in easy accessibility and reduction the cost associated with the administrative and manual data maintenance operations in clinical trials. The growth of the Electronic Trial Master File in healthcare market is attributed to the increasing in number of clinical trial, increasing prevalence of diseases and technology advancement have been boosting the market over the years. However, dearth of skilled professionals is likely to have a negative impact on the growth of the market in the coming years. On the other hand, increasing strategic initiatives by market players is likely to provide growth opportunities over the coming years.
The Electronic Trial Master File in healthcare market is expected to witness substantial growth post-pandemic. The COVID-19 has affected economies and industries in various countries due to lockdowns, travel bans, and business shutdowns. The COVID-19 crisis has overburdened public health systems in many countries and highlighted the strong need for sustainable investment in health systems. As the COVID-19 pandemic progresses, the healthcare industry is expected to see a drop in growth. The life sciences segment thrives due to increased demand for invitro diagnostic products and rising research and development activities worldwide. However, the medical technologies and imaging segment is witnessing drop in sales due to a smaller number of surgeries being carried out and delayed or prolonged equipment procurement. Additionally, virtual consultations by healthcare professionals are expected to become the mainstream care delivery model post-pandemic. With telehealth transforming care delivery, digital health will continue to thrive in coming years. In addition, disrupted clinical trials and the subsequent delay in drug launches is also expected to pave the way for entirely virtual trials in the future. New technologies such as mRNA is expected to emerge and shift the pharmaceutical industry and market is also expected to witness more vertical integration and joint ventures in coming years.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONElectronic Trial Master File (eTMF) Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Growing Applications of Electronic Trial Master File in Healthcare to Drive Electronic Trial Master File in Healthcare Market Growth
Research & development (R&D) is a significant and essential part of a company's business. The operations of the pharmaceutical industry have significant socio-economic impacts on society in the form of R&D and manufacturing investments. The research & development is the "backbone" of any drug discovery system to success, and the electronic trial master file is an essential software in research and development of new pharmaceutical and biotechnology based therapeutic entities. Pharmaceutical and biotech companies majorly focuses on research and development (R&D) to come up with new molecules for various therapeutic applications with the most significant medical and commercial potential. The companies invest majorly in the R&Ds intending to deliver high quality and innovative products to the market. For instance, Global R&D spending in 2017 increased by 3.9 percent to $165 billion compared to 2016. The average R&D expenditure increased moderately to 20.9 percent as a percentage of total prescription sales. Additionally, according to an annual survey of members of PhRMA in 2017 pharma companies reported spending of $71.4 billion on research and development.
The Pharmaceutical companies invested more in R&D to ramp up their clinical trial process. For instance, as of June 30, 2019, AstraZeneca blazed the path by spending 25.63% of revenues on research and development, as of March 31, 2019, Holding strong, Eli Lilly and Company spent 22.38% of its revenues on R&D, and as of June 30, 2019, Roche Holding AG wasn't far behind with 21.29% spent on R&D.
Research and development expenditures are usually incurred during processes of discovering, testing, and developing new products, upfront payments, and milestones, improving existing outcomes, as well as demonstrating product efficacy and regulatory compliance before launch. Moreover, investments for R&D by pharmaceutical companies in the US had grown consistently over the last 15 years.
Drug development and discovery is a time-consuming and expensive process. The process from early detection or design to development to regulatory approval can take more than 10 to 15 years. Throughout the development phase of a drug substance, various testing services are required to check the quality and efficacy of the product. Hence, the pharmaceutical and biotech companies prefer to save the data into electronic master file to save the cost and time, which is expected to drive the growth of the market.
Clinical trials are one of the most important and significant step in drug discovery whether the treatment, medical strategy, or device is safe and effective for human as well as veterinary use. Clinical studies help to understand and determine the best approaches to treatment for certain fields of therapy. Clinical trials are performed specifically to gather data about the safety and efficacy of the development of a new product and tool. Before the regulatory authorities approve the drug molecules and medical devices, a series of clinical studies are carried out. The increasing prevalence of various communicable and non-communicable diseases are increasing the demand for the development of new drugs or medical devices for the treatment. This in turn is expected to increase the demand for clinical trial activities for various therapeutic areas.
Biopharmaceutical and pharmaceutical companies involved in clinical trials aims to shift from paper-based document management systems in file cabinets to electronic document management systems where documents are stored in electronic archives online. By implementing a comprehensive eTMF system that allows organizations to automate, capture and manage TMF documents and records unnecessary risk and can often realize clinical trial cost savings over manual paper handling processes.
A rise in the adoption of an electronic trial master file system in the clinical process likely to boost the market. For instance, NextDocs by Aurea Software is an electronic trial master file (eTMF) forum for clinical cooperation in the management of clinical trial records. It is a content management system for the pharmaceutical industry which offers a formalized means of organizing and storing documents, photographs, and other digital content for clinical pharmaceutical trials that may be required to comply with government regulatory agencies. In clinical trials, EMA fully supports the use of eTMF systems for electronic storage as a substitute for paper. The agency cites quality problems with TMFs and eTMFs in a cautionary statement due to paper content and inconsistencies such as missing pages, unsuitable labeling, or incomplete documents. Because of innovative features of eTMF such as centralization and management of clinical trial documentation, powerful search capabilities (with Metadata), and multiple methods of adding documents, eTMF is becoming important for business efficiency, cost savings, and shortened timelines for the production of BioPharma products to implement electronic document management processes. The secret to introducing interoperable eTMF frameworks is the use of a common content model; vocabulary-based standards; and web-based standards technologies.
Due to advancement in trial master file to electronic trial master file, the contract research organization and pharmaceutical & biotechnology companies are adopting the eTMF for better clinical data management and clinical trial management process. The above mentioned reasons and factors are owing to boost the growth of electronic trial master file market.
Component-Based Insights
In terms of component, the Electronic Trial Master File in healthcare market is segmented into service, and software. The service segment held the largest share of the market in 2019.
Delivery Mode -Based Insights
Based on delivery mode, the Electronic Trial Master File in healthcare market is segmented into cloud-based etmf, and on-premise etmf. The cloud-based eTMF segment held the largest share of the market in 2019.
End User-Based Insights
In terms of end user, the Electronic Trial Master File in healthcare market is segmented into pharmaceutical and biotechnology companies, cros, and others. The pharmaceutical and biotechnology companies segment held the largest share of the market in 2019.
The Electronic Trial Master File in healthcare market players are adopting the product launch and expansion strategies to cater to changing customer demands worldwide, which also allows them to maintain their brand name globally.
Electronic Trial Master File (eTMF)
Electronic Trial Master File (eTMF) Market Regional InsightsThe regional trends influencing the Electronic Trial Master File (eTMF) Market have been analyzed across key geographies.
Electronic Trial Master File (eTMF) Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2018 | US$ 938.32 Million |
| Market Size by 2027 | US$ 3,155.64 Million |
| Global CAGR (2018 - 2027) | 16.5% |
| Historical Data | 2016-2017 |
| Forecast period | 2019-2027 |
| Segments Covered |
By Component
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Electronic Trial Master File (eTMF) Market Players Density: Understanding Its Impact on Business Dynamics
The Electronic Trial Master File (eTMF) Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Electronic Trial Master File in Healthcare Market – by Component
- Service
- Software
Electronic Trial Master File in Healthcare Market – by Delivery Mode
- Cloud-Based eTMF
- On-Premise eTMF
Electronic Trial Master File in Healthcare Market – by End User
- Pharmaceutical and Biotechnology Companies
- CROs
- Others
Electronic Trial Master File in Healthcare Market – by Geography
-
North America
- US
- Canada
- Mexico
-
Europe
- France
- Germany
- Italy
- UK
- Spain
- Rest of Europe
-
Asia Pacific (APAC)
- China
- India
- South Korea
- Japan
- Australia
- Rest of APAC
-
Middle East & Africa (MEA)
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
-
South America and Central America (SCAM)
- Brazil
- Argentina
- Rest of SCAM
Company Profiles
- Aurea, Inc.
- Transperfect.
- Covance Inc (Lab Corp)
- Oracle
- Ennov
- Mastercontrol, Inc.
- Omnicomm
- Pharmavigilalnce
- Veeva Systems
- Phlexglobal
Frequently Asked Questions
Ankita is a dynamic market research and consulting professional with over 8 years of experience across the technology, media, ICT, and electronics & semiconductor sectors. She has successfully led and delivered 100+ consulting and research assignments for global clients such as Microsoft, Oracle, NEC Corporation, SAP, KPMG, and Expeditors International. Her core competencies include market assessment, data analysis, forecasting, strategy formulation, competitive intelligence, and report writing.
Ankita is adept at handling complete project cycles—from pre-sales proposal design and client discussions to post-sales delivery of actionable insights. She is skilled in managing cross-functional teams, structuring complex research modules, and aligning solutions with client-specific business goals. Her excellent communication, leadership, and presentation abilities have enabled her to consistently deliver value-driven outcomes in fast-paced and evolving market environments.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For