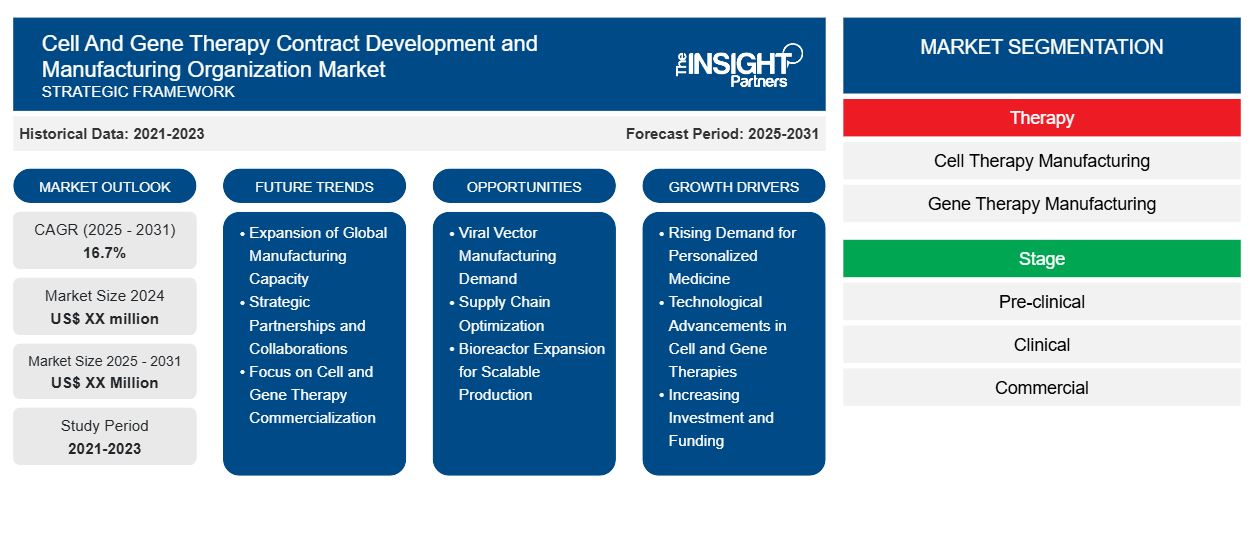
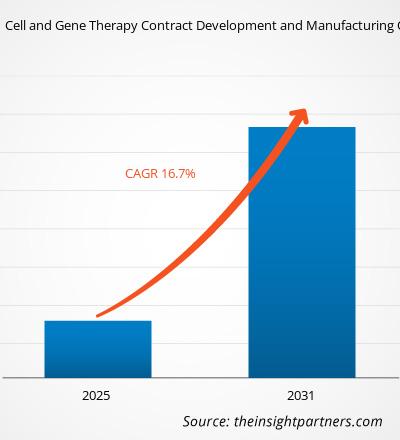
细胞和基因治疗合同开发与制造组织市场规模预计将从2024年的62.2亿美元增至2031年的318.6亿美元。预计2025年至2031年期间,该市场的复合年增长率将达到26.4%。人工智能与数字化转型的日益融合可能会在预测期内带来新的市场趋势。
细胞和基因治疗合同开发和制造组织市场分析
细胞和基因疗法需要专门的生产工艺,包括病毒载体、转导细胞和其他专用生物材料的生产。这些疗法针对的是罕见或复杂的疾病,例如遗传性疾病、癌症和自身免疫性疾病。根据再生医学联盟 (ARM) 的数据,截至 2024 年,仅针对基因疗法的临床试验总数就已超过 1,000 项,另有数百项正在筹备中,这催生了对合同开发和生产组织 (CDMO) 的需求。
越来越多的生物技术初创公司和生物制药公司进入基因和细胞治疗领域,推动了对CDMO的需求。生物制药公司,尤其是中小型企业,缺乏生产这些特殊疗法所需的基础设施和专业知识。因此,他们寻求CDMO的帮助,寻求其在管理临床试验生产、确保合规性和扩大生产流程方面的全面专业知识。Autologus Therapeutics与AGC Biologics Milan的合作始于2020年,当时该公司参与了Autolus的obe-cel CAR-T候选产品AUCATZYL的病毒载体的开发、生产和供应。双方的合作对于该疗法的及时上市起到了至关重要的作用。2025年5月,Astraveus SAS与荷兰干细胞和基因治疗临床发展中心(NecstGen)建立战略合作伙伴关系,以评估Lakhesys台式细胞工厂在CAR-T疗法生产中的应用。
人工智能 (AI) 等技术进步优化了临床试验的生产流程。这些创新促成了高效且经济的生产方法,鉴于所涉及的复杂性,这一点至关重要。因此,创新疗法临床试验的增多,以及对 CDMO 的需求不断增长,推动了细胞和基因治疗合同开发与制造组织市场的增长。
细胞和基因治疗合同开发和制造组织市场概览
随着创新疗法临床试验的增多以及监管审批和商业化进程的加速,细胞和基因疗法合同开发与生产组织市场正在不断扩大。市场中的知名企业正致力于创新和协作,以增强产品的可用性和覆盖范围。2025年4月,AGC Biologics成立了全新的专门的细胞和基因业务部门。新的细胞和基因技术部门将专注于提升AGC Biologics现有的能力,并为需要产能、科研能力以及技术合格的细胞和基因CDMO运营商的开发商提供支持。AGC Biologics米兰细胞和基因卓越中心将成为该新部门的中心。该中心拥有30年的细胞和基因疗法经验,已获得9项商业审批,并成功生产了数百个GMP批次产品。然而,细胞和基因疗法合同开发与生产组织的高度制造复杂性阻碍了市场的增长。
定制此报告以满足您的要求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
细胞和基因治疗合同开发和制造组织市场:战略洞察
-
获取此报告的顶级关键市场趋势。此免费样品将包括数据分析,从市场趋势到估计和预测。
细胞和基因治疗合同开发和制造组织市场驱动因素和机遇
监管审批和商业化激增
过去几年,美国食品药品监督管理局 (FDA) 和欧洲药品管理局 (EMA) 简化了细胞和基因疗法的审批流程。这一快速流程使创新疗法能够更快地进入市场,并增加了对CDMO提供的专业生产服务的需求。这一变化的主要原因之一是人们认识到细胞和基因疗法在治疗先前无法治愈的疾病方面具有重要作用。FDA 推出了再生医学先进疗法 (RMAT) 认定和突破性疗法认定,以加快有前景的疗法的开发和审查。这些认定有助于加快细胞和基因疗法的审批。用于治疗脊髓性肌萎缩症(SMA) 的基因疗法 Zolgensma 于 2019 年首次获得 FDA 批准。到 2024 年,它将在短短四年内在其他 51 个国家获得批准,而这一过程通常需要更长的时间。此次快速审批体现了监管机构推动将改变人生的疗法推向市场的动力。据再生医学联盟 (ARM) 称,自 2020 年以来,全球已有超过 24 种基因疗法获得监管部门批准,还有更多疗法正在筹备中。
使用专业设施和技术
细胞和基因疗法的需求正推动生物制药公司寻求能够提供尖端设施和技术的CDMO,这些设施和技术是扩大生产规模、确保质量、合规性和获得监管批准的必要条件。这些专业能力对于复杂和个性化疗法的生产至关重要,而这些疗法需要先进的基础设施来维持高生产标准。细胞和基因疗法,包括基因编辑、病毒载体生产和个性化医疗,需要配备最新技术的专门设施。基因疗法中使用的病毒载体的生产需要符合GMP标准的设施,以确保最终产品的安全性、一致性和质量。这些设施应能够在受控和监控的环境中处理活细胞和转基因生物。自动化细胞培养系统、连续生产和数字化质量监控系统的日益普及,提高了基因疗法生产的增长和效率。2025年3月,Bharat Biotech公司在印度南部投资7500万美元,建立了其首个细胞和基因治疗设施。预计该设施将在未来3年内推出针对肿瘤和罕见疾病的新疗法。
对专业设施的需求为CDMO创造了巨大的机遇。对于生物制药公司来说,与拥有所需技术和设施的CDMO合作既经济高效,又能节省成本。2023年,百时美施贵宝与一家CDMO合作生产其CAR-T细胞疗法Breyanzi。该疗法涉及采集、改造和扩增患者的T细胞,这一过程需要专业的技术和设施才能确保达到预期的治疗效果。通过利用CDMO在专业设施方面的专业知识,百时美施贵宝得以在确保合规性和质量的同时扩大生产规模。因此,对先进制造能力和先进技术的不断增长的需求预计将为细胞和基因治疗合同研发组织市场创造未来的增长机会。
细胞和基因治疗合同开发和制造组织市场报告细分分析
有助于得出细胞和基因治疗合同开发和制造组织市场分析的关键部分是服务类型、产品类型和最终用户。
- 根据服务类型,细胞和基因治疗合同开发与制造组织市场细分为药物开发与制造、检测与监管服务以及其他服务。药物开发与制造领域在2024年占据了最大的市场份额。
- 就产品类型而言,细胞和基因治疗合同开发与制造组织市场分为基因治疗和细胞治疗。2024年,细胞治疗领域占据市场主导地位。
- 根据最终用户,细胞和基因治疗合同开发与制造组织市场可分为制药公司、生物制药公司和其他公司。2024年,生物制药公司占据了市场主导地位。
细胞和基因治疗合同开发和制造组织市场份额(按地区)分析
细胞和基因治疗合同开发和制造组织市场报告的地理范围主要集中在五个地区:北美、亚太地区、欧洲、南美和中美、中东和非洲。就收入而言,北美在 2024 年占据了市场主导地位。预计在预测期内,它将继续在全球市场占据主导地位。美国生物技术日新月异,遗传病患病率不断上升,对专业制造服务的需求激增。根据美国政府问责局 2021 年 10 月发布的估计,美国约有 2500 万至 3000 万人患有罕见病。根据美国食品药品监督管理局 (FDA) 的数据,超过 7000 种罕见病影响着该国超过 3000 万人。对这些疾病的了解日益加深,导致基因治疗发展激增。CDMO 通过为针对罕见遗传病的基因疗法的开发和制造提供专业服务,在这一领域发挥着至关重要的作用。
2023年,美国FDA批准了众多细胞和基因疗法,包括针对罕见病的基因编辑疗法。用于治疗镰状细胞病的exagamglogene autotemcel(Casgevy)和lovotibeglogene autotemcel,以及用于治疗重症血友病A的valoctocogene roxaparvovec等疗法均已获得FDA批准,凸显了基因疗法在应对罕见病挑战方面的潜力。再生医学先进疗法(RMAT)认证等加速的监管途径,促使生物技术公司与CDMO合作,以扩大生产规模。
制造业基础设施的投资促进了市场的增长。由15家学术机构、超过25家公司和政府机构合作成立的国家细胞制造联盟 (National Cell Manufacturing Consortium) 旨在实现细胞疗法的经济高效、大规模生产。此外,CDMO、学术机构和生物制药公司之间的战略合作也促进了市场的增长。
细胞和基因治疗合同开发和制造组织市场区域洞察
Insight Partners 的分析师已详尽阐述了预测期内影响细胞和基因治疗合同开发与制造组织市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的细胞和基因治疗合同开发与制造组织市场细分和地域分布。
- 获取细胞和基因治疗合同开发和制造组织市场的区域特定数据
细胞和基因治疗合同开发和制造组织市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2024年的市场规模 | 62.2亿美元 |
| 2031年的市场规模 | 318.6亿美元 |
| 全球复合年增长率(2025-2031) | 26.4% |
| 史料 | 2021-2023 |
| 预测期 | 2025-2031 |
| 涵盖的领域 |
按服务类型
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
细胞和基因治疗合同开发和制造组织市场参与者密度:了解其对业务动态的影响
细胞和基因治疗合同开发与制造组织市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求的驱动因素包括消费者偏好的演变、技术进步以及对产品优势的认知度的提升。随着需求的增长,企业正在扩展产品线,不断创新以满足消费者需求,并抓住新兴趋势,从而进一步推动市场增长。
市场参与者密度是指特定市场或行业内企业或公司的分布情况。它表明特定市场空间内竞争对手(市场参与者)的数量相对于其规模或总市值而言。
在细胞和基因治疗合同开发和制造组织市场运营的主要公司有:
- 药明生物技术有限公司
- 查尔斯河实验室国际公司
- 康泰伦特公司
- 龙沙集团
- 赛默飞世尔科技公司
- AGC Biologics AS
免责声明:以上列出的公司没有按照任何特定顺序排列。
- 获取细胞和基因治疗合同开发和制造组织市场顶级关键参与者概述
细胞和基因治疗合同开发和制造组织市场新闻和最新发展
细胞和基因疗法合同开发与制造组织市场评估是通过收集一手资料和二手资料后进行的定性和定量数据进行的,这些数据包括重要的企业出版物、协会数据和数据库。以下是细胞和基因疗法合同开发与制造组织市场的主要发展:
- 药明生物推出EffiX微生物表达平台,助力重组蛋白和质粒DNA生产。(来源:药明生物,2025年3月)
- Charles River Laboratories International, Inc. 与 AAVantgarde 宣布达成 CDMO 协议,以生产符合良好生产规范 (GMP) 的质粒 DNA。AAVantgarde 是一家临床阶段生物技术公司,拥有两个专有的腺相关病毒 (AAV) 载体平台,用于大基因递送,并致力于开发治疗遗传性视网膜疾病的产品。该公司将利用 Charles River 在生产符合良好生产规范 (GMP) 的质粒 DNA 方面的专业知识。(来源:Charles River Laboratories International, Inc.,2024 年 7 月)
- 通用型AAV免疫基因癌症疗法的先驱Siren Biotechnology与Catalent Inc.建立战略合作伙伴关系,后者是全球领先的AAV免疫基因疗法研发和生产供应商,致力于为患者提供更优质的治疗方案。(来源:Catalent Inc.,新闻稿,2024年5月)
细胞和基因治疗合同开发和制造组织市场报告覆盖范围和交付成果
《细胞和基因治疗合同开发和制造组织市场规模和预测(2021-2031)》报告对以下领域进行了详细的市场分析:
- 伤口闭合装置市场规模及全球、区域和国家层面所有主要细分市场的预测
- 伤口闭合装置的市场趋势以及市场动态,例如驱动因素、限制因素和关键机遇
- 详细的 PEST 和 SWOT 分析
- 伤口闭合装置市场分析涵盖主要市场趋势、全球和区域框架、主要参与者、法规和最新市场发展
- 行业格局和竞争分析,涵盖市场集中度、热图分析、知名参与者以及细胞和基因治疗合同开发和制造组织市场的最新发展
- 详细的公司简介
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 细胞和基因治疗合同开发和制造组织市场
获取免费样品 - 细胞和基因治疗合同开发和制造组织市场