Cell Therapy Bioprocessing Market Analysis and Emerging Trends by 2034
Cell Therapy Bioprocessing Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Bioreactor, Lyophilization, Electrospinning, Control flow Centrifugation, Ultrasonic Lysis, Genome Editing Technology, Cell Immortalization Technology, Viral Vector Technology); Cell Type (Stem Cell, Immune Cell, Human Embryonic Stem Cell, Pluripotent Stem Cell, Hematopoietic Stem Cells); Indication (Cardiovascular Disease (CVD), Oncology, Wound Healing, Orthopedic, Others); End user (Hospitals and Clinics, Diagnostic Centers, Regenerative Medicine Centers, Academic and Research Institute)
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Apr 2026
- Report Code : TIPRE00021550
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
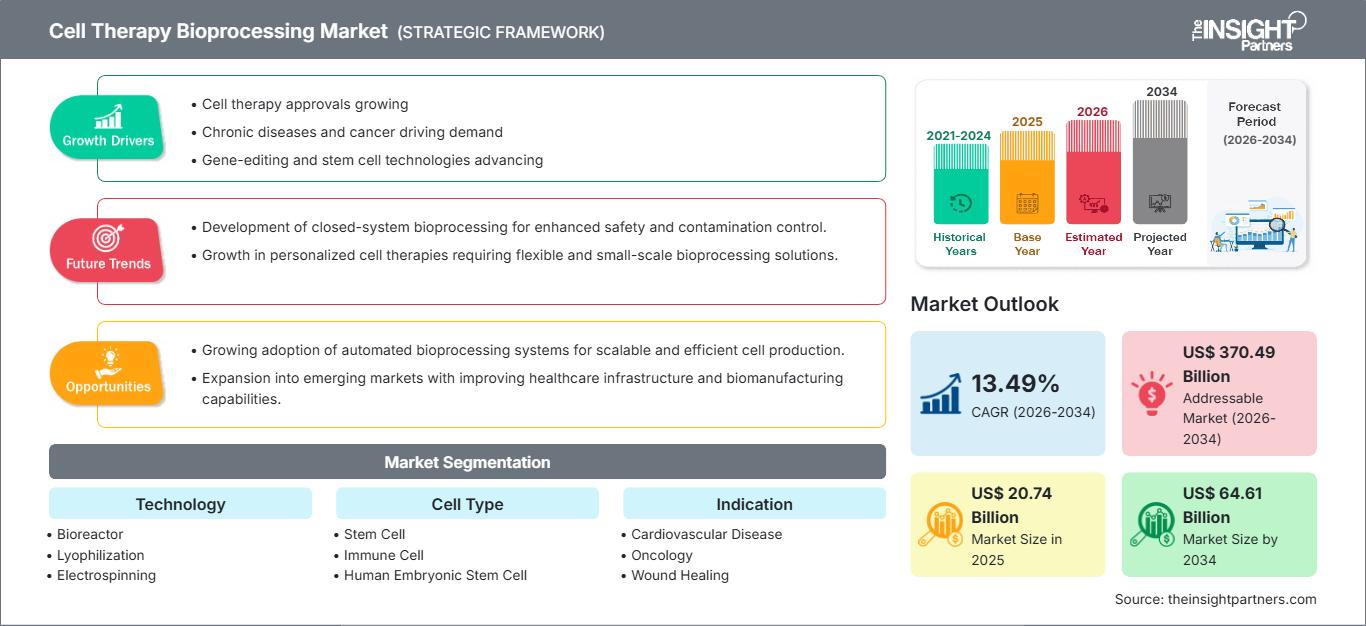
The cell therapy bioprocessing market size is expected to reach US$ 64.61 billion by 2034 from US$ 20.74 billion in 2025. The market is anticipated to register a robust Compound Annual Growth Rate (CAGR) of 13.49% during the forecast period of 2026–2034.
Cell Therapy Bioprocessing Market Analysis
The cell therapy bioprocessing market forecast indicates robust, multi-faceted growth, primarily driven by the transition of cell and gene therapies from niche treatment modalities to mainstream clinical application. The market expansion is facilitated by critical technological advancements, including the widespread adoption of single-use bioreactors for increased flexibility, the integration of automation and robotics to minimize human error and scale production, and the utilization of AI-driven analytics for real-time process monitoring and optimization. Furthermore, increasing investments in regenerative medicine, combined with accelerating regulatory support (such as Fast Track designations for novel therapies), are rapidly pushing therapeutic candidates through clinical trials and into commercial production, thereby strengthening the market's trajectory.
Cell Therapy Bioprocessing Market Overview
Cell therapy bioprocessing involves the complex end-to-end workflow required for the development and manufacturing of therapeutic cells, including mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and various immune cells (e.g., CAR-T cells) for clinical use. The core processes are highly demanding and must strictly adhere to Good Manufacturing Practice (GMP) conditions. The bioprocessing workflow typically encompasses several critical stages: cell isolation from source material, large-scale cell expansion and culture to achieve therapeutic dose, harvesting and purification of the final product, and highly specialized preservation and cryo-storage for distribution. The market is currently undergoing a significant shift from costly, patient-specific autologous therapies toward industrialized, cost-effective allogeneic (off-the-shelf) therapies, which demands more scalable and standardized manufacturing platforms.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONCell Therapy Bioprocessing Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Cell Therapy Bioprocessing Market Drivers and Opportunities
Market Drivers:
-
Rising Prevalence of Chronic and Life-Threatening Diseases: The increasing global incidence of chronic diseases, particularly various types of cancer (driving demand for CAR-T and adoptive T-cell therapies) and severe cardiovascular disorders, creates an urgent, growing demand for innovative therapeutic alternatives like cell therapies.
-
Surge in R&D Investments and Government Funding: Significant private and public funding, including large-scale government initiatives aimed at supporting regenerative medicine and advanced therapy medicinal products (ATMPs), fuels research, clinical trials, and the necessary infrastructure development for bioprocessing technologies.
-
Technological Shift to Closed and Automated Systems: The move towards automated, closed bioprocessing systems, which reduce contamination risk and enhance process efficiency and standardization, is critical for achieving the scalability required for commercialization.
Market Opportunities:
-
Expansion into Emerging Markets and CDMO Services: Growth in developing economies (like those in the Asia-Pacific) with expanding healthcare infrastructure and rising patient awareness offers vast, untapped markets. Additionally, the increasing reliance on specialized Contract Development and Manufacturing Organizations (CDMOs) for commercial-scale production presents major service-based opportunities.
-
Integration of Process Analytical Technology (PAT): Incorporating Artificial Intelligence and digital twins for process modeling, predictive maintenance, and real-time Quality Control (QC) offers substantial opportunities to optimize yields, reduce failure rates, and lower the overall Cost of Goods Sold (COGS).
-
Standardization of Allogeneic Manufacturing Platforms: The industrialization of allogeneic therapies requires highly scalable, robust bioprocessing solutions, creating a substantial opportunity for manufacturers to develop and market standardized, high-throughput manufacturing platforms.
Cell Therapy Bioprocessing Market Report Segmentation Analysis
The market is analyzed across several critical segments that define the industry structure and focus areas for investment. Below is the standard segmentation approach used in most industry reports:
By Technology:
-
Bioreactor
-
Lyophilization
-
Electrospinning
-
Control flow Centrifugation
-
Ultrasonic Lysis
-
Genome Editing Technology
-
Cell Immortalization Technology
-
Viral Vector Technology
By Cell Type:
- Stem Cell
- Immune Cell
- Human Embryonic Stem Cell
- Pluripotent Stem Cell
- Hematopoietic Stem Cells
By Indication:
-
Cardiovascular Disease
-
Oncology
-
Wound Healing
-
Orthopedic
By End User:
-
Regenerative Medicine Centers
-
Academic & Research Institutes
-
Hospitals & Clinics
-
Diagnostic Centers
By Geography:
-
North America
-
Europe
-
Asia Pacific
-
South & Central America
-
Middle East & Africa
Cell Therapy Bioprocessing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 20.74 Billion |
| Market Size by 2034 | US$ 64.61 Billion |
| Global CAGR (2026 - 2034) | 13.49% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Technology
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Cell Therapy Bioprocessing Market Players Density: Understanding Its Impact on Business Dynamics
The Cell Therapy Bioprocessing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Cell Therapy Bioprocessing Market Share Analysis by Geography
The market demonstrates varying rates of maturity and growth across regions, heavily influenced by regulatory frameworks, public funding, and the concentration of biotech expertise. Below is a summary of market share and trends by region:
-
North America
-
Market Share: Holds the largest share, driven by an established, advanced healthcare and R&D ecosystem.
-
Key Drivers: High concentration of major pharmaceutical and biotech firms, strong regulatory environment (e.g., FDA's accelerated approval pathways), and substantial venture capital funding for cell therapy startups.
-
Trends: Rapid adoption of fully automated and digitalized bioprocessing solutions, and a growing network of specialized CDMOs focusing on commercial-scale CAR-T manufacturing.
-
-
Europe
-
Market Share: Commands a significant share, backed by strong public funding and consolidated biotech hubs.
-
Key Drivers: Favorable European Union (EU) funding programs (e.g., Horizon Europe), streamlined regulatory frameworks for ATMPs (Advanced Therapy Medicinal Products), and a highly active academic research environment.
-
Trends: Increasing focus on establishing harmonized, multi-site manufacturing networks to handle cross-border clinical trials and commercial supply across EU member states.
-
-
Asia-Pacific (APAC)
-
Market Share: Projected to be the fastest-growing region, led by China and India.
-
Key Drivers: Rapidly increasing government initiatives to boost domestic life science and biotech sectors, expansion of healthcare infrastructure, a large, rapidly aging patient population, and cost advantages in manufacturing.
-
Trends: Heavy investment in establishing GMP manufacturing facilities, strong focus on adopting cutting-edge, cost-effective automation technology, and accelerating local clinical trial activity.
-
-
South & Central America and Middle East & Africa (SCA & MEA)
-
Market Share: Emerging markets with significant growth potential.
-
Key Drivers: Increasing investments in modernizing healthcare infrastructure, growing awareness, and preliminary regulatory steps toward adopting cell therapies, and public-private partnerships focusing on medical innovation.
-
Trends: Early adoption of readily available, scalable technologies like closed single-use systems to establish initial bioprocessing capabilities quickly.
-
Cell Therapy Bioprocessing Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
The market is characterized by intense competition among established life science tool providers and specialized cell therapy solution vendors.
Major companies, including Thermo Fisher Scientific Inc., Sartorius AG, and Lonza, leverage their deep expertise in bioprocessing equipment and CDMO services to offer integrated solutions across the entire cell therapy value chain. Competition drives continuous innovation, with vendors focusing on:
-
Closed System Development: Creating highly efficient, closed, and automated systems to reduce contamination risk and manual labor.
-
Strategic Partnerships: Collaborating with clinical-stage biotech companies to secure long-term supply agreements for reagents and equipment.
-
Facility Expansions: Increasing global manufacturing capacity, particularly in specialized viral vector production and cell processing services (CDMOs).
Major Companies Operating in the Cell Therapy Bioprocessing Market
-
Thermo Fisher Scientific Inc.
-
Sartorius AG
-
Lonza
-
Merck KGaA
-
Cytiva
-
Corning Incorporated
-
Fresenius Kabi AG
-
Asahi Kasei Corporation
-
Repligen Corporation
Disclaimer: The companies listed above are not ranked in any particular order.
Cell Therapy Bioprocessing Market News and Recent Developments
-
Thermo Fisher introduced OpTmizer One SFM, a specialized serum-free medium formulated to significantly boost T‑cell expansion and maintain the early memory T‑cell phenotype, a critical quality attribute for effective cell therapies.
-
In October 2025, Miltenyi Biotec announced it will commercially supply its lentiviral vectors (LVV) for Immatics' anzutresgene autoleucel (anzu-cel, IMA203), showcasing its expanding CDMO role and reliable supply chain for critical raw materials.
-
Lonza reinforced its cell & gene therapy expertise by offering a comprehensive, end-to-end process development service. This spans the entire therapeutic lifecycle, from early-stage Investigational New Drug (IND) filing support all the way through commercial GMP manufacturing.
Cell Therapy Bioprocessing Market Report Coverage and Deliverables
The "Cell Therapy Bioprocessing Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering the areas listed below:
-
Cell Therapy Bioprocessing Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope.
-
Cell Therapy Bioprocessing Market trends, as well as market dynamics such as drivers, restraints, and key opportunities.
-
Detailed PEST and SWOT analysis.
-
Cell Therapy Bioprocessing Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments.
-
Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the Cell Therapy Bioprocessing Market.
-
Detailed company profiles.
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For